Approaches To Best Practice MES Implementation
By Paul Murray, senior consultant, Enterprise System Partners
Traditionally, Manufacturing Execution Systems (MES) implementations, including Master Batch Records (MBR) modelling, have been executed by project teams located on site owing to the complex nature of the projects. However, as MES becomes better understood by project stakeholders, there are opportunities to consider other options. In this article, we examine the approaches available to pharmaceutical organizations to deliver a successful project.
The Traditional Approach
Once a decision has been reached to implement MES, the fundamental question to address is if you want to develop these resources from your existing team, hire in new resources with specialist skills or use a third party integration company to support this need?
Typically, MES implementations have been executed by project teams located on-site.
The main factors influencing the decision to perform work on site are:
- MES projects impact many if not all site functions
- MES projects are usually complex
- Definitions, interpretations or expectations of MES systems remain many and varied
- MES vendors and integrators are typically still learning what best practices are applicable to various plant types. This requires a high level of interaction with the client
- There may be reluctance on the part of the client to relinquish control, based on the cost and complexity of the project
Typically implementations can be divided into three key phases: Application Layer Development, Modelling Configuration, and Operational Rollout.
Application Layer Development
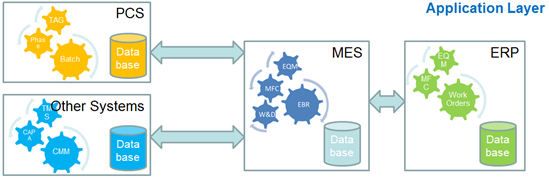
The “Application Layer” includes the base MES application and database, interfaces primarily to ERP and Automation, and other customizations if required. These elements are typically developed installed, qualified and validated as a core build by a client Centre of Excellence (CoE) team. Business resources are required from representative sites for this phase mainly for requirements definition with most of the work being done by a combination of System Vendor, CoE and Site IT teams.
A large involvement from the vendor is required at this stage as the development of the interfaces and customizations impacts directly on the MES itself. These should be delivered by the vendor to ensure that they have no adverse impact and that they are compatible with any future system upgrades and the product roadmap.
CoE and Site IT resources will need to be familiar with the MES Application Layer and the interfaces to be in a position to support the system when the vendor work is complete.
50% of the overall budget costs can be consumed during this phase, which includes the licensing costs of the MES system. Given the current maturity of MES Systems the technical risks are relatively minimal. However, the business risks remain significant due to the impact of an error, or omission of a key requirement, across many sites.
Once deployed on site, the core build is ready for development of the site specific modelling configuration.
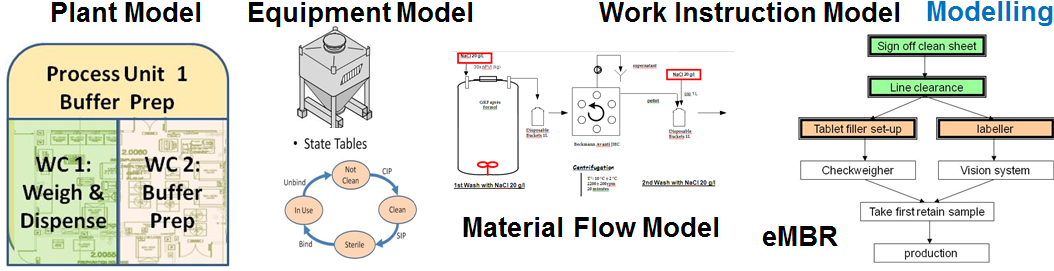
Modelling Configuration:
The modelling is the development of the plant process within the MES which typically falls into Master Data and Master Batch Record (MBR) Configuration. Master Data includes the products, material definition and flow, equipment definition and work center locations, while the MBR includes workflow, work instructions and data collection.
The development of this model has a very significant impact on the subsequent efficient operation of the plant. It effectively determines the benefits which the MES will bring when implemented.
If this model is not optimally developed, it may result in the full potential of the MES system not being realized.
While MES trained business resources are essential to bring the business knowledge to the effort, given the rich and complex functionality now available as standard in MES systems (e.g. complex equipment state diagrams, parameterized and object oriented MBR’s etc.), it is challenging to design and develop an efficient model without using specialist and experienced modelling resources. Specialist vendor resources may be needed to bring deeper understanding of the complex, and new, functionality available after system upgrades.
Specialist modelling resources, who understand both the system capabilities and the requirements of a pharmaceutical manufacturing environment, as well as having a background in structured computer programming, are normally required to develop such models. These modelling specialists typically bring best practice in this area as they will have likely encountered similar process in previous projects.
The absence of specialist modellers runs the risk of the development of a model that does not promote the most efficient configuration and may be inflexible in dealing with future products and plant expansions.
The long term efficiency of the plant is determined by the effectiveness of the initial model developed at this point. Errors made in the modelling at this stage can be very costly to remedy retrospectively once an operating history is established.
Generally the best model, which produces the greatest long term benefits to operations, is developed by a combination of business resources and specialist modellers.
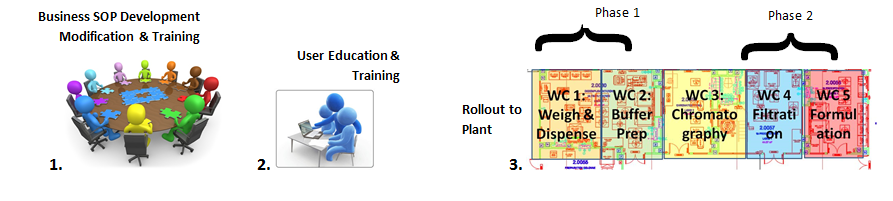
Operational Rollout
Once modelling is completed and fully validated, the operational rollout of the system to the plant can begin. The operational rollout, with its requirement for smooth and phased cutover with minimal disruption to operations, is also best carried out by business resources supported by a specialist project management team who have previous experience and know the issues involved in a site wide training and rollout program.
The phasing of the rollout can be a complex process involving not only user training but also development or revision of Standard Operating Procedures (SOPs) that govern the MES and the manufacturing process, as well as all the User Workstations and associated peripherals required to run the system. In most cases, the initial rollout is performed in tandem with the existing paper based process to minimize risk, until sufficient usage have been performed without any system issues.
Where possible, the rollout should be designed in progressive stages, preferably with areas of lowest risk being cut-over first - to give the MES a good starting reputation with the user community.
It is desirable to have a specialist team to plan and execute the rollout in an efficient manner with minimal disruption to plant throughput and ensure an adequate provision of hyper care during the initial go live to ensure success.
Off-Site MES Work Practice
A recent development in the implementation of MES systems in the life science industry is to take more and more of the development work off-site from the plant, typically using a third party integrator where the majority of work is carried out by a team based off-site. The key drivers behind this are:
- Decrease cost while increasing quality: MES projects have become complex and costly. If executed correctly, this model offers control and opportunity to simplify and standardize, reducing cost and improving quality of outputs.
- Find highly qualified resources: Utilize highly specialist MES resources with minimum travel & living time and expense burdens. Conventional site work does not always attract the best resources.
- Life cycle support that is affordable: Establish a core team to support the MES system life cycle instead of providing supplemental resources who are gone once the project is over.
- Deliver on time & within budget: Fixing scope under this model enables these essential requirements to be met.
Site work is still required in this model and mainly involves requirements and design workshops and final on site system testing - with all the system modelling, documentation and functional testing taking place off site. Typically, on previous projects executed using this model, the offsite-onsite split is 80:20.
After initial client/integrator requirements process mapping, the integrator takes the bulk of the work off-site to model or configure governed by a defined scope. Development is interspersed by periodic visits to the site for additional workshops to further define requirements, review design prototypes and complete dry runs.
Once complete, the modelling is reviewed and full design documentation is issued to the client for approval. When the design is approved, test protocols can be developed remotely, approved by the client, and functional test execution performed off site. Off-site testing is then completed, fully approved and can proceed to final Site Acceptance Testing (SAT). On successful completion, the system is handed over to the client together with the required support from the integrator.
The project is generally fixed fee with a tightly defined scope, and must have robust project management controls to ensure the cost and schedule benefits are delivered.
Activities are generally carried out directly on client servers via VPN access or it is carried out on the vendors/integrators servers and the build exported once modelling activities are complete.
One of most significant and challenging barriers to this approach is giving up direct control of the project to a third party, so it is crucial to:
- engage an integrator who is proficient and reliable with substantial project implementation experience in relevant industry sectors
- ensure adequate business resources are available for requirements definition and design review
- have robust project management and change control procedures in place
The principal disadvantage to this model is that site personnel do not get the level of exposure to modelling and early stage testing. This means they are less familiar with the components once the system is ready to go live. To counteract that, sufficient training time should be factored into your budget and schedule, along with the option for site resources to engage in prototype dry runs during the modelling configuration phase.
There is also the risk that client expectations may not be aligned with the build, given the remote development. This is mitigated with regular prototype reviews during the modelling process.
Conclusion
While the traditional model of using site based resources is still the most prevalent approach still used in the life science industry, more and more companies are using specialist resource teams based off-site to achieve improved quality and standardization of MBRs while reducing the cost of delivery. There are challenges to overcome, not least individual sites giving up direct control of their project. These can all be overcome using trusted specialist resources, fixing scope and applying appropriately tight project controls.
