India's New Drugs, Medical Devices, And Cosmetics Draft Bill 2022
By Mathini Ilancheran, principal analyst, R&D, Beroe Inc.

To accommodate changing requirements and encourage the adoption of new technology, India’s Ministry of Health and Family Welfare released a draft bill in July 2022 to replace the existing Drugs and Cosmetics Act 1940. This act governs drug importation, production, and distribution across the country. The act's main goal is to ensure that all drugs and cosmetics sold in India are safe, effective, and meet international standards.1 It also focuses on ensuring the quality, safety, efficacy, and performance of new drugs, as well as on the clinical investigation of investigational medical devices. Finally, it adds regulations and restrictions on drug imports aimed at protecting public health interests. Below are some of the areas most highly impacted by this new bill.
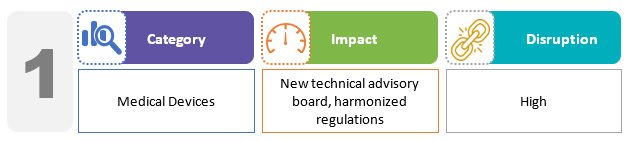
The bill proposes setting up a medical device technical advisory board and opens medical device testing facilities in states and at the federal level, similar to drug laboratories. The bill significantly expands the regulations governing medical device quality. In particular, the manufacture and import of medical devices that do not meet the prescribed standards are specifically prohibited. The draft bill aims to uniformly regulate studies for all classes of medical devices.2,3
The new bill, in conjunction with Medical Devices (Amendment) Rules 2020, results in a regulatory framework for medical device manufacturing that is distinct from that for drugs and medicines. To carry out any manufacturing, distribution, sale, or importing of a medical device in India, all medical device manufacturers must register with a government-designated portal. This registration process would necessitate the submission of the company's name and address, as well as the addresses of the manufacturing sites, device details, ISO 13485 certificates, and so on.4 The bill also aims to reduce India's reliance on imported high-end medical devices to less than 30% compared to the present 80%. Proposals include tax breaks and refunds to encourage medical device exports, increased government spending on high-risk medical device projects, and a single-window clearance system for licensing medical devices.5
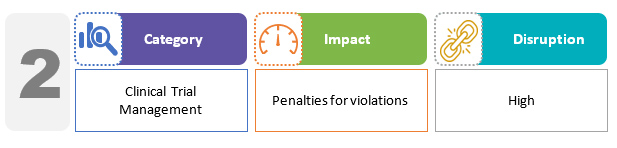
The bill includes requirements to obtain permission before conducting clinical research, as well as medical management and compensation for study-related injury and death. Specific penalties for violations of these provisions have been proposed. Academic trials and biomedical research are specifically excluded from compliance under the CT rules.2,3
For manufacturers, the bill seeks to regulate clinical trials for medical devices by defining investigational medical devices and establishing related provisions for clearances, inspections, penalties, and compensation. These new regulations will ensure that clinical studies are conducted in a transparent and safe manner.
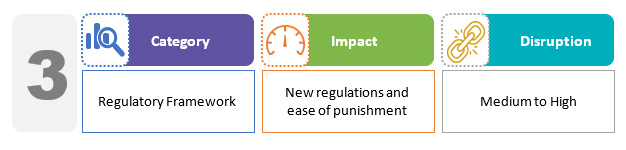
The Drugs, Medical Devices, and Cosmetics Consultative Committee (DMDCCC) is a new body for drugs and medical devices. The Drugs Controller General of India chairs this committee, which advises various governments and related parties on matters pertaining to ensuring uniformity in the administration of drugs and medical devices throughout the country. The draft bill, in particular, establishes the procedure for improvement notices. The licensing authorities may issue an improvement notice outlining compliance measures or directing the correction of an existing violation. This allows the licensee to correct any violations rather than the licensing authority directly initiating proceedings for licence condition violations.2,3
Though all offenses under the new draft bill have higher penalties compared to the previous regime, the bill also addresses the pharmaceutical industry's request to decriminalize some of the existing law's offenses. For example, the government has proposed lowering the penalties for not of standard quality (NSQ) drugs.6 All of the above is to ensure harmonization with international standards and achieve an improved regulatory process.
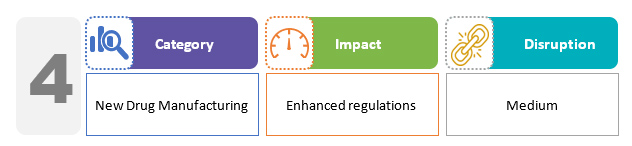
With respect to new drug manufacturing, there is a prohibition in place for the production of any new drug without a license. The provisions for drug regulation in the bill do not deviate significantly from the existing regime. However, the penalties for violations under the draft bill are harsher than under the current regime.2,3
These new regulations, along with the New Drugs and Clinical Trials Rules, 2019 issued by the Central Drugs Standard Control Organization (CDSCO), will ensure more transparency and expedition in new drug approvals, especially orphan drugs for rare diseases, as well as new innovative medicines that have already been approved and licensed by regulators in the EU, U.S., Australia, Canada, and Japan.
Conclusion
The flaws in the 1940 Drugs and Cosmetics Act revealed a poor regulatory framework for medical devices. Hence, the new bill focuses on regulating medical devices as a distinct category. It also acknowledges the role of independent governing authorities with knowledge of medical devices. The objective is to make medical devices one of the key sectors, increasing India's share in the global medical device industry from $11 billion to $50 billion in the next 10 years. However, there is still a lack of clarity on the Medical Device Reporting (MDR), which is a post-market tool for monitoring device performance. Similarly, it does not address post-marketing drug surveillance or recall. In conclusion, the intent of the new draft bill is to improve quality, increase consumer trust, and stakeholder expectations.
References
- N. Siddiqui and C. A. Samuel, "What’s new in the Drugs, Medical Devices and Cosmetics Bill, 2022?," August 2022. [Online]. Available: https://www.barandbench.com/columns/whats-new-in-the-new-drugs-medical-devices-and-cosmetics-bill-2022.
- D. Punnen and D. M. Antani, "Draft Drugs, Medical Devices and Cosmetics Bill, 2022: Dawn of a New Era?," Nishith Desai Associates, August 2022. [Online]. Available: https://www.natlawreview.com/article/draft-drugs-medical-devices-and-cosmetics-bill-2022-dawn-new-era. [Accessed October 2022].
- MINISTRY OF HEALTH & FAMILY WELFARE, "DRAFT OF NEW DRUGS, MEDICAL DEVICES AND COSMETICS BILL.2022," DEPARTMENT OF HEALTH & FAMILY WELFARE, July 2022. [Online]. Available: https://main.mohfw.gov.in/newshighlights-97. [Accessed October 2022].
- A. Patel, "How regulatory changes for medical devices sector are impacting manufacturers," Financial Express, November 2021. [Online]. Available: https://www.financialexpress.com/healthcare/medicaldevices/how-regulatory-changes-for-medical-devices-sector-are-impacting-manufacturers/2362650/. [Accessed October 2022].
- S. Barik, "Explained: India’s draft medical devices policy, and why it is needed," Indian Express, March 2022. [Online]. Available: https://indianexpress.com/article/explained/india-draft-medical-devices-policy-explained-7820757/. [Accessed October 2022].
- P. Gupta, "New drugs Bill: Govt must balance needs of pharma business with public interest," Policy Circle, August 2022. [Online]. Available: https://www.policycircle.org/industry/new-drugs-bill-eodb-pharma/. [Accessed October 2022].
About The Author:
 Mathini Ilancheran is the principal analyst of R&D for Beroe Inc. She specializes in understanding market scenarios across the globe in the outsourcing arena. Her analysis has enabled global fortune 500+ companies in their strategic decisions on supply base optimization, risk reduction, innovation and efficient sourcing. She has written for several publications related to R&D procurement opportunities. With her category knowledge, she has published 35+ articles in leading journals, co-authored with industry experts. She has a master's in management from University College London (UCL) and has worked as a consultant in the U.K. You can contact her at mathini.ilancheran@beroe-inc.com and connect with her on LinkedIn.
Mathini Ilancheran is the principal analyst of R&D for Beroe Inc. She specializes in understanding market scenarios across the globe in the outsourcing arena. Her analysis has enabled global fortune 500+ companies in their strategic decisions on supply base optimization, risk reduction, innovation and efficient sourcing. She has written for several publications related to R&D procurement opportunities. With her category knowledge, she has published 35+ articles in leading journals, co-authored with industry experts. She has a master's in management from University College London (UCL) and has worked as a consultant in the U.K. You can contact her at mathini.ilancheran@beroe-inc.com and connect with her on LinkedIn.
