Mission TransCelerate: Transforming The Drug Development Terrain
By Rob Wright, Chief Editor, Life Science Leader
Follow Me On Twitter @RfwrightLSL
 During the ill-fated 1970 Apollo 13 mission to the moon, it was astronaut Jack Swigert who alerted ground control that something had gone terribly wrong when he uttered the phrase, “Houston, we’ve had a problem here.” Those same words seem very fitting to the current state of affairs around the skyrocketing costs of drug discovery. Recent estimates place the expense of successfully bringing just one drug to market at between $350 million and $1.2 billion. However, in the last decade, companies having brought 4 to 13 drugs to market have watched the price tag reach stratospheric heights — orbiting $5 billion+. “I think the pain point has reached a threshold that’s no longer bearable,” states Dalvir Gill, Ph.D., CEO of TransCelerate BioPharma.
During the ill-fated 1970 Apollo 13 mission to the moon, it was astronaut Jack Swigert who alerted ground control that something had gone terribly wrong when he uttered the phrase, “Houston, we’ve had a problem here.” Those same words seem very fitting to the current state of affairs around the skyrocketing costs of drug discovery. Recent estimates place the expense of successfully bringing just one drug to market at between $350 million and $1.2 billion. However, in the last decade, companies having brought 4 to 13 drugs to market have watched the price tag reach stratospheric heights — orbiting $5 billion+. “I think the pain point has reached a threshold that’s no longer bearable,” states Dalvir Gill, Ph.D., CEO of TransCelerate BioPharma.
A nonprofit organization founded in September 2012 by 10 member companies, TransCelerate set out on a bold mission — to collaborate across the global pharmaceutical and biotech R&D community to identify, prioritize, design, and implement solutions to simplify and accelerate the delivery of innovative new therapies. This is easier said than done in an industry with a history of companies working in secret, racing to be first to market. Now totaling 18 (see Table 1) participants, TransCelerate members include private, public, and VC-backed companies, ranging in age from a little over one year to nearly 300, and hailing from Japan, the EU, and the United States. With combined annual revenues in excess of $300 billion and nearly 800,000 employees worldwide, the prospect of TransCelerate being successful must be similar to imagining the United States and the former Soviet Union actually collaborating to put a man on the moon during the height of the Cold War. And yet, having only been in existence a little over a year, TransCelerate is transforming the drug development terrain faster than many thought possible. Dr. Gill, a 25-year drug development veteran, reveals the biggest roadblock to TransCelerate’s success and how it was overcome. In addition, he explains the important role organizational structure plays in driving results, as well as the science behind the initiative selection process.
The Simple Key To Overcoming Skepticism
“When I first heard about the TransCelerate opportunity, it was a scary proposition,” recalls Gill. Skeptical about if it would actually work, he began gathering insight into the organization’s mission and leadership commitment. As he did, the former president of Phase 2 to 4 drug development at PharmaNet-i3 (now known as inVentiv Health) began to believe that it could not only work, but it had to work. “The drug development industry was running out of options. We had to find a way to collaborate to remove drug development inefficiencies,” Gill affirms. Reflecting on his decision to “seize” the TransCelerate opportunity, he says, “I took on this position, and I have not regretted it for one day. How often does a person encounter an opportunity in their career where taking a slightly different, perhaps riskier, path can have such massive implications on bringing more medicines to people?” The path was riskier because Gill was walking away from a successful career. In addition, the path would be much more difficult, because nothing as bold as the TransCelerate initiative had ever been attempted before in the clinical space.
 Some may think sending a man to the moon to be difficult. However, discovering the cure for cancer or Alzheimer’s is more difficult. Otherwise, it would have already been done. Many are skeptical if we will ever find cures for these kinds of diseases. Skepticism proved to be one of the biggest roadblocks to TransCelerate gaining liftoff. “The history of these kinds of not-for-profit, precompetitive collaborations has yielded mixed results, at best,” he asserts. According to Gill, the key to overcoming skepticism is getting results. “As TransCelerate quickly started to produce and publish tangible, pragmatic, actionable, deliverables (e.g. its risk-based monitoring paper at the end of May 2013), some of the initial skepticism started to dissipate. Next came the announcement of a plan for a common clinical-site qualification and training initiative. This was followed by TransCelerate delivering on other successful initiatives. Thus, the key to overcoming the initial skepticism was delivering results. But none of that would have been possible without an organizational structure that encourages and enables its members but also holds them accountable for delivering results.
Some may think sending a man to the moon to be difficult. However, discovering the cure for cancer or Alzheimer’s is more difficult. Otherwise, it would have already been done. Many are skeptical if we will ever find cures for these kinds of diseases. Skepticism proved to be one of the biggest roadblocks to TransCelerate gaining liftoff. “The history of these kinds of not-for-profit, precompetitive collaborations has yielded mixed results, at best,” he asserts. According to Gill, the key to overcoming skepticism is getting results. “As TransCelerate quickly started to produce and publish tangible, pragmatic, actionable, deliverables (e.g. its risk-based monitoring paper at the end of May 2013), some of the initial skepticism started to dissipate. Next came the announcement of a plan for a common clinical-site qualification and training initiative. This was followed by TransCelerate delivering on other successful initiatives. Thus, the key to overcoming the initial skepticism was delivering results. But none of that would have been possible without an organizational structure that encourages and enables its members but also holds them accountable for delivering results.
Results Orientation Requires That Form Follow Function
TransCelerate’s organizational structure, which had been put in place prior to his arrival, was one of the reasons Gill was attracted to the position. “A big contributor to why consortiums fail is because they often don’t have leadership at the right level,” states Gill. In his experience, consortium projects are frequently managed and run by consultants and third-party providers. As a result, company people do not get deeply involved, and initiatives never really take off. To prevent this from happening at TransCelerate, the configuration was set up to involve member-company leaders at the highest levels and in a structure similar to a pharmaceutical company. For example, Gill reports to chairman of the board Paul Stoffels, CSO and worldwide chairman of pharmaceuticals for Johnson & Johnson. The organization has a board of directors representing each member company. “At the board level, we have some very senior-level leaders, some responsible for multibilliondollar R&D budgets,” he affirms. “They have accountability within the TransCelerate organization and need to be on board with how we operate, the projects we pick, and progress being made.” According to Gill, they are consulted regularly through board meetings and other interactions. The operations committee, which also consists of senior-level people from member companies, handles the day-to-day running of the business.
“When you have these two layers of top-level support, you have the ability to move projects along quickly,” says Gill. “More importantly, when results and deliverables are released, these individuals have the ability to drive implementation as opposed to having a nice white paper that people can pin up on their bulletin board.”
In addition to having the right leadership, Gill stresses the importance that prioritization has played in TransCelerate’s success. For example, once the organization gains approval to commission a project, it is treated within member companies like any other internal project. “We look for project leadership that makes sense, staffing the team with people from the member companies who fit with the right subject-matter expertise,” he states. From there, the team drafts its time line, deliverables, and budget, refining the project from the original proposal. “These folks are held accountable to deliver to TransCelerate,” Gill explains. “These projects should not be secondary priorities.”
Gill recognizes that maintaining prioritization of TransCelerate projects is challenging for member employees, since they have their day jobs to contend with, too. That’s why part of the budgeting process includes assigning fulltime equivalent (FTE) contributions to indicate the necessary member-employee workload for a project. In some cases, the FTE is as high as 100 percent being dedicated toward a TransCelerate project. “Twenty-five to 30 percent is at the bottom end of the FTE commitment allocation,” Gill shares. TransCelerate employs only two full-time employees; the rest of the work is completed by member-company personnel who define problems and come up with solutions. This strategy not only creates buy-in and accountability, it prevents projects from languishing. “We don’t get calls saying, ‘Hey, this project slipped by for three weeks, and we are sorry about that,’” Gill says. “People realize that’s not an acceptable response, and they will have to justify these things to me and the entire operations committee.”
The Science Of Project Selection
At its core, TransCelerate was created to cut through the red tape and duplicated work so often associated with skyrocketing R&D drug development costs. But the scope of such an endeavor was daunting, with potential projects including everything from improving efficiencies in collaborations, clinical/preclinical data sharing, and target validation. The initial potential-project list numbered 30. From there, TransCelerate leadership began the process of narrowing the focus to a more manageable number. To do this, member companies sent some of their best people to apply a tactical approach to project selection. “Initially we wanted to pick doable, tangible, quick-hitting projects that had enormous value and a return on investment from multiple perspectives (e.g. reductions in dollars or FTEs) and for multiple stakeholders,” he says. This process narrowed the list to 10 projects.
The next step involved conducting project-feasibility analyses to determine how much effort each project would require in terms of money, manpower, and expertise. The projects under consideration were also assessed from an intangible perspective (e.g. value to trial participants, higher safety). From that data, TransCelerate built a project road map. “If a project was foundational to the road map — meaning that to complete project ‘B’ we must first finish project ‘A’ — it got higher priority than a project that might deliver some immediate value but was not necessarily going to move the overall ‘landscape’ forward as quickly as we would like,” Gill explains. Through this process, the list was narrowed to five projects for initial action: (1) risk-based monitoring, (2) site qualification and training, (3) clinical data standards, (4) comparator drugs, and (5) shared-investigator portal. Each project also included a targeted outcome. For example, the site-qualification-and-training initiative included target outcomes of common criteria for mutual recognition of GCP training and common forms to collect generic information about study sites. Of the initial five projects, TransCelerate has delivered actionable information on all but one — the shared-investigator portal.
Though Gill is proud of all of TransCelerate’s accomplishments, he is most impressed with the success of the comparator-drug initiative. This is because it had the highest degree of skepticism as to its feasibility. “Very few people believed competitive companies would facilitate access to comparator drugs from each other for use in comparator clinical trials,” he says. “Once the facts were presented, people realized companies almost always secured comparator drugs; it was just a matter of how painful we wanted to make the process.” According to Gill, people began to understand the logical benefits this network would achieve. Companies would be able to launch trials faster. Acquiring drugs would cost less. Most importantly, studies would be safer by avoiding all the issues around drug stability and the possible inadvertent acquisition of counterfeit drugs. On Aug. 6, 2013, TransCelerate announced the successful establishment of the clinical trial comparator network and the initiation of its first transaction.
To look at future projects, TransCelerate created the aptly named future initiatives team. Representatives of this team evaluate the current state of clinical trials and postulate what technology, process, or regulatory changes will occur in three to five years. The team then creates a road map detailing how to achieve the clinical trial state of the future. Gill says this strategic process was used to help determine the prioritization and fit of the recently announced additional TransCelerate initiatives: (1) common clinical trial protocol template, (2) special-populations clinical trial networks (pediatric and minority), and (3) investigator registry. Gill provides an example of why linking current and future initiatives is not only important but logical. “One of the current projects involves developing data standards in collaboration with CDISC [Clinical Data Interchange Standards Consortium] and the FDA. If we are standardizing data for certain therapeutic areas, it makes sense to start tackling the front end of the process, which is the protocol for those therapeutic areas.” This is why TransCelerate launched the common-protocol template initiative, which will match therapeutic areas with agreed-upon data standards for each therapeutic area. “Similarly, if you have a shared-portal platform, it needs an investigator registry that allows unique identification, credentialing, and identity management for clinical trial investigators around the world,” Gill contends.
In my discussion with Gill, he revealed how his performance as CEO of TransCelerate is measured — delivery on announced initiatives, organization operations, and organization governance. When asked how it has been going thus far, he replied, “I think overall we have made really outstanding progress this year. We have delivered on the projects we said we were going to deliver on.” Interestingly, about six months prior to writing this article, I was contacted by a reader proposing the submission of a TransCelerate “call-to-action” article. They expressed how TransCelerate wasn’t moving quickly enough. While I have yet to receive a rough draft of the proposed paper, during the same time period, TransCelerate has delivered results on four of its five initial projects and added three new ones, as well. Perhaps all consortiums should aspire to move as slowly as TransCelerate.
The Keys To Building A Successful Consortium
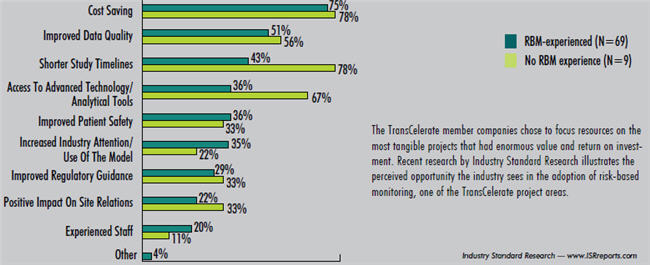
For the founding 10 TransCelerate BioPharma companies, the benefits of membership were very clear (e.g. reducing redundant costs). In addition, the structure provides members the autonomy to select (if any) which initiatives to implement at their organizations. For example, a survey conducted by Industry Standard Research (see graph) reveals a variety of reasons to adopt risk-based monitoring, one of TransCelerate’s initial initiatives. However, just because you are a TransCelerate member does not mean you are required to implement the outcomes resulting from every initiative tackled. According to TransCelerate CEO Dalvir Gill, Ph.D., this flexibility is just one of the keys to building a successful consortium.
Adequate funding is another key. But at TransCelerate, it is not the type of funding you might imagine. “We are not an organization that is funded by a bucketful of money,” he states. “Our funding is a fraction of a fraction of what funds many consortiums.” TransCelerate’s funding primarily comes in the form of time and intellect. “The member company defines a problem and then puts some of their best brains on coming up with a solution,” he explains.
Finally, Gill suggests another key to creating a successful consortium is to create it so that it can easily be dissolved when no longer needed. He refers to this as project sustainability. “My job as CEO is to plan for all of our projects to be sustainable with or without the existence of TransCelerate.” However, given the number of projects currently being undertaken, Gill suspects the organization won’t be closing its doors anytime soon.
There could be many reasons to adopt risk-based monitoring.
Which of the following do you think are increasing the adoption the most? Select all that apply.
The Keys To Building A Successful Consortium
When Dalvir Gill joined TransCelerate as its CEO, he admits that some of the work in creating the company’s structure was already in place. Currently, there are only two full time employees and he believes having such a minimalistic infrastructure is one of the keys to creating a successful consortium. “Many consortiums overbuild their operations infrastructure, spending more money on operations than actual deliverables. That’s not our plan,” he affirms.
Gill’s TransCelerate office is located within part of a GSK facility, which reduces overhead. As a former employee of a CRO, he understands the value of outsourcing noncore competencies. “We outsource everything — auditing, legal, IT — anything we can,” he explains.
Adequate funding is another key to creating a successful consortium. But at TransCelerate, it is not the type of funding you might imagine. “We are not an organization that is funded by a bucketful of money,” he states. “Our funding is a fraction of a fraction of what funds many consortiums.” Finally, Gill suggests another key to creating a successful consortium is designing it in such a way that it can easily be dissolved when no longer needed. He refers to this as project sustainability. “My job as CEO is to plan for every one of the projects that we are doing to be sustainable with or without the existence of TransCelerate.”
Given the number of projects currently being tackled by TransCelerate, Gill suspects the organization probably won’t be closing its doors anytime soon.
The Benefits Of Participating In TransCelerate Go Beyond Its Own Initiatives
For the original 10 companies that had the vision to get involved in creating TransCelerate, the benefits were very clear (e.g. reducing redundant costs). But member-company employees found additional benefits, as well. “Our remit is to look at improving efficiency of drug development via collaboration in the precompetitive space,” states TransCelerate’s CEO, Dalvir Gill. “That being said, there are some additional side benefits for those who participate.” For example, every two weeks, key leaders from the operations committee meet and talk about progress on TransCelerate projects. “These people and these companies are becoming familiar with one another, allowing them to collaborate on non-TransCelerate matters far more easily than they would have otherwise,” he reveals.
In addition to bringing leaders of competing companies together, TransCelerate also works closely with trade organizations, such as the Association of Clinical Research Organizations (ACRO) and the Society for Clinical Research Sites (SCRS), providing these organizations with progress updates. Though TransCelerate’s remit isn’t the creation of networking opportunities among industry leaders, it may be this side benefit which really makes the difference. By providing a forum for industry leaders to not just meet, but actually collaborate on meaningful projects, precompetitive silos that existed between companies are being dismantled, creating a wealth of opportunities to accelerate drug development beyond the initiatives being tackled by TransCelerate.
