Practical Application Of Online Water Bioburden Analyzers In Pharmaceutical Manufacturing
By Hans-Joachim Anders (Novartis), Fred Ayers (Eli Lilly), Brian Fitch (Biogen), Ren-Yo Forng (Amgen), Scott Hooper (Merck & Co.), Michelle Luebke (Baxter), Jeanne Mateffy (Amgen), Peter Noverini (Baxter), Brian Termine (GSK), Lisa Yan (Shire), and Jeffrey Weber (Pfizer Inc.) of the Online Water Bioburden Analyzer Workgroup
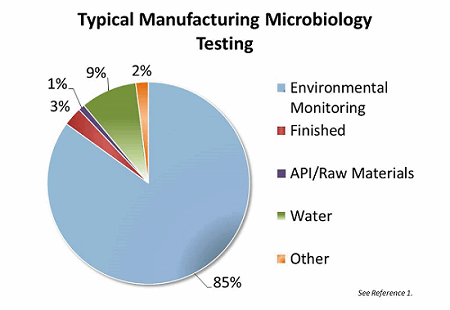 Water is the single largest quantity raw material used in pharmaceutical manufacturing. Water is used in all facets of manufacturing, from preparing equipment to manufacturing the final commercial product. Grab-sample testing of that water constitutes approximately 9 percent of the total testing volume in a typical aseptic manufacturing facility.1 Pharmaceutical water needs to be routinely monitored and controlled for conductivity, total organic carbon, bioburden, and endotoxin (based on use of the water). Currently, bioburden monitoring is conducted by periodic manual grab sampling, plating, and traditional microbiological growth, a process that requires several days to complete. The goal of online water bioburden analyzers is to provide real-time continuous information of the water systems and thereby provide real-time risk reduction through enhanced process resolution and understanding.
Water is the single largest quantity raw material used in pharmaceutical manufacturing. Water is used in all facets of manufacturing, from preparing equipment to manufacturing the final commercial product. Grab-sample testing of that water constitutes approximately 9 percent of the total testing volume in a typical aseptic manufacturing facility.1 Pharmaceutical water needs to be routinely monitored and controlled for conductivity, total organic carbon, bioburden, and endotoxin (based on use of the water). Currently, bioburden monitoring is conducted by periodic manual grab sampling, plating, and traditional microbiological growth, a process that requires several days to complete. The goal of online water bioburden analyzers is to provide real-time continuous information of the water systems and thereby provide real-time risk reduction through enhanced process resolution and understanding.
Current online water bioburden analyzers are designed using novel technology that makes use of the intrinsic biochemical auto-fluorescence of microorganisms upon laser excitation. Current commercial systems use a laser (typically 405 nm) to excite natural biological molecules (e.g. NADH and picolinic acid) and detect the resulting emitted fluorescence. This technique can be used for both air and liquid samples where the moving sample stream passes through an optical cell and the analyzer continuously monitors the detection frequency, particle size, and bio-fluorescence. The opportunity to use bio-fluorescence particle counting to provide continuous, real-time signals of changes in the air or water loops in manufacturing areas provides significant preventive and process-diagnostic value. Recent articles have described alternative or rapid microbial methods for water testing that omitted the bio-fluorescence-based analyzers.2 The intrinsic fluorescent-based systems provide an analytical result based on auto fluorescent units versus the colony-forming units of growth-based traditional methods.3 While an OWBA is not currently a panacea allowing elimination of all traditional testing (though progress is being made), the technology provides several practical applications for current pharmaceutical manufacturing.4
Risk Reduction Roles For Online Water Bioburden Analyzers
There are several practical applications for an OWBA that should not require changes to registrations or pre-approval meetings, unless previous commitments have been made. These roles provide enhanced process understanding and risk reduction. This approach has widely been used for the evaluation of generic process analytical technology (PAT) in manufacturing, where processes are monitored to understand the variability in water generation and distribution system.5 A primary benefit of an OWBA is continuous real-time monitoring of water systems. This provides a vastly superior approach to continuous monitoring, trending, and alarming as compared with periodic grab sampling and utilizing long incubation times.
Listed below are some known applications for OWBA systems that are proposed or are in current use on pharmaceutical water systems.
Water Hold Time Extensions
Many manufacturing sites have relatively short water hold times based on the initial system validations. While many systems could extend the hold times based on sampling and testing, there is minimal financial benefit for individual tanks due to the labor and testing costs involved. An OWBA provides on-going system health monitoring, thereby allowing extension of hold times without sampling or laboratory labor and material costs. Although water is relatively low cost, the benefit of replicating this approach to increase hold times is typically approximately $9,000 per tank annually;6 therefore, the benefits rapidly increase as the method is implemented across a facility.
Robust Documentation Of System Equilibrium Restoration
Another role for OWBA units is to provide continuous monitoring of water loops that have undergone maintenance or are challenging to maintain in a state of control. The OWBA removes the variability of manual sampling to enable the qualified utilities group to focus on true shifts in the water generation system’s reliability and to enable enhanced testing during periods of adverse trends. The OWBA provides additional information for appropriate intervals of maintenance activities such as change of reverse osmosis (RO) membranes or change of softener resin; some companies, including our site, discard the water after an RO change until the results of the traditional bioburden testing results are available. OWBA units can be used to verify the effectiveness of loop sanitization cycles and frequencies to robustly ensure that the water is fit-for-purpose prior to use. In this application, the OWBA is installed at a sampling point downstream from the location of the maintenance or the critical control point. Comparison of pre- and post- maintenance data sets demonstrate the system has been returned to a state of control. An example would be four hours of monitoring prior to maintenance and calculating the average and standard deviation and comparing this with the values after maintenance and cleaning. The confidence gained from the continuous OWBA data may allow companies to return back to manufacturing rapidly based on a risk assessment of the system being examined.

Need to update your current facility to include new aseptic technology? Get the ABC's of facilities enhancement in the webinar:
Aging Aseptic and Biological Manufacturing Facilities – Renovation for Survival.
Biofilm Surveillance
Biofilms, if detected, are difficult to characterize, can be missed via periodic grab sampling, and are a widespread threat to purified water systems. Sites that suspect the presence of biofilms have challenges in developing effective identification and remediation strategies. One approach is to install an OWBA near the suspect area and cause turbulence to loosen any potential films; this can be either through intentionally creating a water hammer or through use of a rubber mallet to strike the piping. This has been demonstrated to release solids (e.g. rouge, sediment, or biofilms) in several cases. The OWBA can be used to quickly provide supportive information of the source, nature, and remediation of issues, rather than waiting for days or even weeks to close out a biofilm investigation.
Superior Monitoring Of Systems
Finally, data integrity has been challenged across the industry. Demonstrating accurate and timely water system information is very difficult for manual water sampling processes. Online water bioburden analyzers provide continuous information to automated data collection systems that can be used to confirm sources of variation in manufacturing processes, whether due to increased particles (e.g. pump failure) or microbial changes in the water system. Many times apparent microbial failures in water systems are assigned to operator error based on inherently weak periodic sampling information. Continuous monitoring provides a deep and robust dataset to fully assess the state of a water system. This fact also allows for robust investigations and points the way to effective corrective and preventive actions (CAPAs).
Conclusions
Roles for online water bioburden analyzers to enable the manufacturing of safe products are gaining acceptance across the industry. Several successful applications have encouraged the industry to pursue a collaborative approach toward the implementation of OWBA systems. The use of the systems to accurately document water system health and changes provides a great support during investigations. The instruments have demonstrated increasing capability and have potential for risk reduction, loop monitoring, and providing insight for investigations.
References:
- “Online Water Bioburden Analyzer Technology Overview,” Jeffrey Weber, PDA podium presentation. May 5, 2015.
- “Microbiological Monitoring of Pharmaceutical Water Systems,” Tim Sandle, European Pharmaceutical Review. Vol. 22, Issue 2, pp 25-27, 2017.
- “Rapid Microbiological Methods and Alternatives to the Colony Forming Unit: Is there a Pathway to Implementation?” Online publication, http://www.pharmaceuticalonline.com/doc/rapid-microbiological-methods-alternatives-to-colony-forming-units-a-pathway-to-implementation-0001. Dec. 7, 2016.
- “Novel Concept for Online Water Bioburden Analysis: Key Considerations, Applications, and Business Benefits for Microbiological Risk Reduction,” Anthony Cundell, Oliver Gordon, Nick Haycocks, Joe Johnston, Michelle Luebke, Neil Lewis, Jeanne Mateffy, and Jeffrey W. Weber, American Pharmaceutical Review. July 2015.
- “Guidance for Industry PAT – A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance,” U.S. Department of Health and Human Services, FDA. September 2004.
- “OWBA: A case study for the extension of purified water hold times,” OWBA Workgroup, Pharmaceutical Engineering. Vol. 35, No. 5, pp 119-124. October 2015.
About The Authors:
The authors are members of a collaborative workgroup formed to guide the development and implementation of an online water bioburden analyzer (OWBA). By leveraging lessons learned through assessment and implementation of various rapid microbiological methods (RMM) and process analytical technologies (PAT) across the healthcare industry, the workgroup has the primary goal of providing guidance regarding the development of OWBA systems that would be broadly accepted by the industry and regulators. To facilitate this goal and engage instrument vendors, the OWBA workgroup has developed three key documents — a user requirements specification (URS), a testing protocol, and a business benefits proposal. The URS provides fundamental OWBA system design inputs, as well as additional desirable attributes. The protocol provides a list of verification and performance tests that the vendor can apply to demonstrate system capability and operation. The business benefit guidance provides the financial justification for both vendors and industry to drive the successful implementation of an OWBA platform.
