Automated Inspection Of Pre-Filled Syringes And Biologics During Fill/Finish
By Tyler Harris
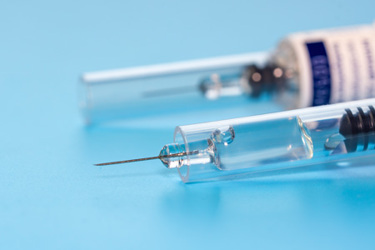
The pre-filled syringe industry is rapidly expanding. Fill/finish is an intricate process requiring many steps in its procedure, from filling and stoppering to labeling and secondary packaging. Often, this intricate process is automated and includes various inspection methods. Commonly, container closure integrity (CCI) testing is left out of the automated process and is either conducted manually with outdated, probabilistic methods or not conducted at all, increasing risk of error.
In any manufacturing process, there is risk. Equipment issues and human error are unavoidable at times. In pharmaceutical production, this error equates to potential safety issues for the patient. Quantitative and deterministic CCI test methods aim to find these errors before reaching the patient, and further mitigate risks to patient safety.
Learn about the requirements of the CCI testing of biologics, two of the most effective scalable technologies for CCI testing, and more.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
