Biopharmaceutical Modular Cleanrooms

Standalone, turnkey, process ready cGMP modular cleanrooms
Germfree cGMP modular cleanrooms are a platform solution that unlock standalone manufacturing capacity for a variety of applications. Serving processes from cell therapy to sterile fill-finish manufacturing cGMP modular facilities offer unmatched finishes, layout flexibility, controls, timeline assurance, and cost benefits for the biopharmaceutical industry. This is why Germfree has been chosen by 12 of the top 20 biopharmaceutical companies as their modular cleanroom providers.
Modular cleanrooms highlights: Biopharma, International building codes, IBC, NFPA, ISO. BSL-2 BMBL, FDA, EMA, cGMP compliant
Applications: Aseptic Filling, Cell Therapy, Gene Therapy, Viral Vector, Plasmids
Turnkey modular cleanrooms to enable cGMP production
Germfree cGMP modular cleanroom facilities are a genuine turnkey platform solution that enable both cGMP and cGMP/BSL-2 production. All systems necessary to operate the facility are fully integrated. Germfree facilities are free standing, do not require a shell building and provide a high quality interior working environment. Germfree Modular facilities are designed with room and facility features such as Grade D (ISO 8), Grade C (ISO 7), Grade B (ISO 5), Material Air Locks (MALs), Personnel Airlocks (PALs), Anterooms, and Degowning rooms in a layout most efficient for your process flow.
- No shell building required
- Independent HVAC design
- Maintained & serviced by Germfree
- Process segregation capabilities
- Adaptable into an existing facility
- Relocatable
Standard Modular cGMP Cleanrooms Layouts
Explore our standard facility layouts that cover a broad range of applications and process flows. If these do not meet your application needs, learn more about our custom solutions.
-
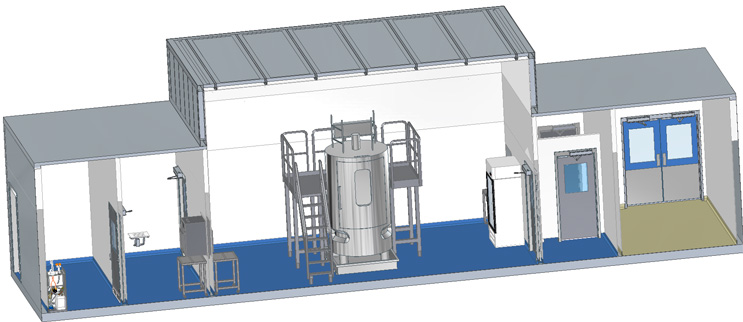
Single Module for Large Process Equipment
-
The flow of this facility contains a CNC Entry Room, Grade D Anteroom, Grade C Processing Suite, Grade D Anteroom, and a CNC Exit Room.
-
-
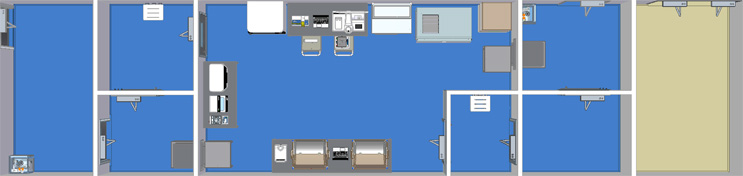
Single Module Large Grade B Unidirectional
-
The flow of this facility contains a CNC entry room, grade D Anteroom, grade C anteroom, large grade B processing suite, grade C anteroom, grade D anteroom and a CNC exit room.
-
-
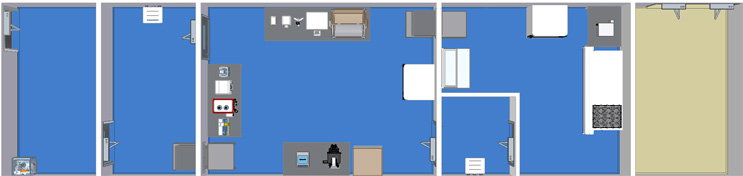
Single Module Large Grade B Suite with Bidirectional Flow
-
This facility flow includes a CNC entryroom, grade D anteroom, large grade C processing suite, grade B anteroom, and a grade B processing suite allowing for a bidirectional flow throughout.
-
-
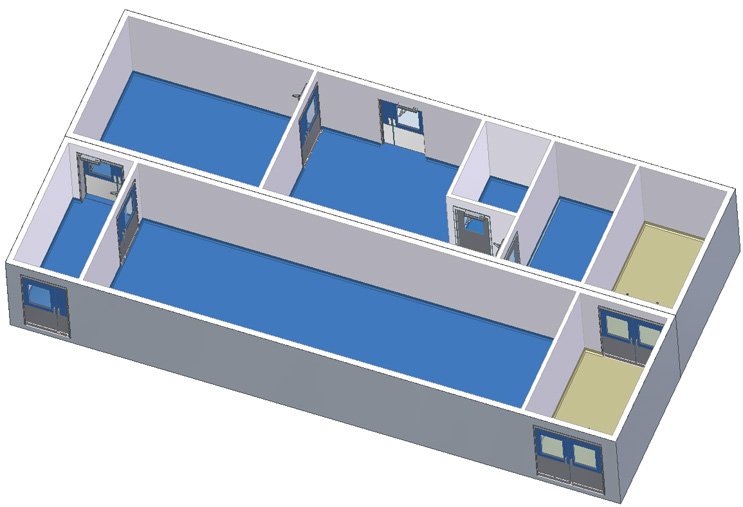
2 Module Facility with Exit Corridor
-
This facility flow is a CNC entryroom, ISO 8/grade D material packing and storage suite, large grade D anteroom connected to a grade D supply corridor with a large grade D suite.
-
-
2 Module Facility with "Ballroom" Layout
-
This facility flow is a CNC entryroom, grade D anteroom, grade C large processing suite suitable for large bioreactors and process equipment with a grade D anteroom and CNC exit.
-

-
5 Module CGT Facility with Unidirectional Flow
-
This 5 module facility has the following flow: CNC entryroom, grade D locker room, grade C gowning and material storage connected to a grade C supply corridor. Large grade C processing suite ballroom, grade D anteroom and a CNC exit corridor.
-

