Claritin patent expiry for Schering-Plough: decongesting the allergies marketplace?
Schering-Plough's Claritin (loratadine) has securely established itself as the world's leading brand allergy product since its launch in 1993. In recent years the product has faced minimal competition reinforcing the monopolistic stronghold of Schering-Plough and the dominance of the antihistamines within the allergic rhinitis market.
The patent expiry of Claritin in December 2002 poses a large threat to Schering-Plough and could signal the end of their dominance in the allergic rhinitis market.
Datamonitor's new report, Market Dynamics 2000: Allergic Rhinitis. Effects of Major Patent Expiries on an Expanding Market, reveals:
- Claritin's patent expiry will have both positive and negative effects on the antihistamines and the allergic rhinitis market
- The drivers behind Claritin's blockbuster status: aggressive product management and marketing, more so than superior drug attributes
Claritin's patent expiry will have both positive and negative effects on the antihistamines and the allergic rhinitis market
Given its dominance of the allergic rhinitis market as a whole, the loss of sales driven by Claritin's patent expiry in December 2002 will have repercussions for both the antihistamine class and the market as a whole. However its not all bad news as the patent expiry of Claritin will open up the allergic rhinitis market, allowing the entry of several new players.
Claritin's success has maintained the antihistamine class as the backbone of allergic rhinitis treatment, despite the shortcomings of the class, which only provides symptomatic relief. The effect of Claritin's patent expiry upon the class will be dramatic and will certainly weaken the antihistamines stronghold on the market. However concerns by manufacturers of other leading brand antihistamines that generic versions of Claritin may flood the market will be allayed by the fact that the allergic rhinitis market is largely driven by brand loyalty, particularly in the US.
A more real threat to the antihistamine class is that Claritin's patent expiry will allow the entry of new drug classes into the allergic rhinitis market. The lack of preventative therapies offering both long-term disease management and the satisfaction of the unmet needs of drug delivery, dosage and safety has created a niche in the market and any novel drug that could address these problems would experience rapid uptake. For the allergic rhinitis market as a whole therefore the news is good, with Claritin's patent expiry promoting innovation and in the long-term leading to an expansion of the allergic rhinitis market.
Datamonitor Analyst Susan Lawlor comments:
"In the long-term the patent expiry of Claritin will breathe new life into the allergic rhinitis market, which is good news for manufacturers and allergy sufferers alike."
The drivers behind Claritin's blockbuster status: aggressive product management and marketing, rather than superior drug attributes
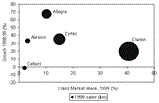
Sales of Schering-Plough's Claritin reached $2.7bn in 1999, accounting for almost a third of sales in the global allergic rhinitis market and giving Schering-Plough over 40% market share of the antihistamine drug class. Claritin's nearest competitor, UCB Pharma's Zyrtec (cetirizine), which is marketed by Pfizer in the US, recorded 1999 sales of less than half that of Claritin.
Datamonitor Analyst Susan Lawlor comments:
"The reason for Claritin's success has been aggressive product management and marketing, more so than superior attributes of the drug itself. In fact several clinical trials have shown that Aventis' Allegra (fexofenadine), launched in 1996, is a more effective antihistamine both in terms of efficacy and side effects. Yet only a small number of patients have switched from Claritin, due to the strength of customer loyalty."
The phenomenal success of Schering-Plough's Claritin can be attributed to a number of factors:
- Schering-Plough's Claritin has grown steadily owing to the addition of several new formulations including Claritin D with added decongestant, a sustained released formulation and most recently the launch of a mouth soluble formulation
- Schering-Plough have actively sought ways to extend Claritin's original patent largely through reformulations and additional indications
- The growth of all Claritin products in the US has been fuelled by an intensive marketing campaign by Schering-Plough, in 1995 the company considerably increased its sales force and begun a direct-to-consumer campaign promoting all forms of Claritin.
In short, the remarkable success of Claritin can be attributed to Schering-Plough's tenacious approach to promoting and maintaining its blockbuster product. However, even Schering-Plough cannot avoid the unavoidable and Claritin's patent expiry is certain to occur in December 2002.

Source: Datamonitor
Editor's Notes
For further information on this press release, please contact:
Elisabeth Overend-Freeman
t) (212) 686-7400 ext. 765
f) (212) 686-2626
Or at:efreeman@datamonitor.com
To register for Datamonitor press services, contact the press office at (212) 686-7400 ext. 765, or visit www.datamonitor.com/press/ to register electronically.
Market Dynamics 2000: Allergic Rhinitis
is available from Datamonitor, priced at $4,600Datamonitor is an independent market analysis firm that publishes a wide portfolio of strategic business information. Datamonitor has expertise in the following industry sectors: Automotive & Transport; Consumer; Financial Services; Healthcare; Industrial; Medical Equipment; Technology. Datamonitor can be contacted at (212) 686-7400 or visit www.datamonitor.com.
