FDA Debuts Pre-Request For Designation Process For Combination Products
By Jof Enriquez,
Follow me on Twitter @jofenriq

The U.S. Food and Drug Administration (FDA) is introducing a new option for device sponsors seeking clarification on how their combination products could be reviewed by the agency.
FDA already provides a mechanism wherein sponsors can submit a Request for Designation (RFD) to the Office of Combination Products (OCP) — to which the agency responds, through a written determination, the product's most appropriate classification. FDA also determines which center ‒ the Center for Devices and Radiological Health (CDRH) for devices, Center for Biologics Evaluation and Research (CBER) for biological products, or the Center for Drug Evaluation and Research (CDER) for drugs ‒ will take the lead in reviewing the combination product.
Sponsors submit an RFD in cases where it's unclear how their product should be classified, or if they anticipate a dispute with FDA over its classification, according to RAPS.
RFD submissions typically contain the product's detailed description, indications for use, composition/ingredients, and an explanation of how it works, as well as input from the relevant FDA center(s).
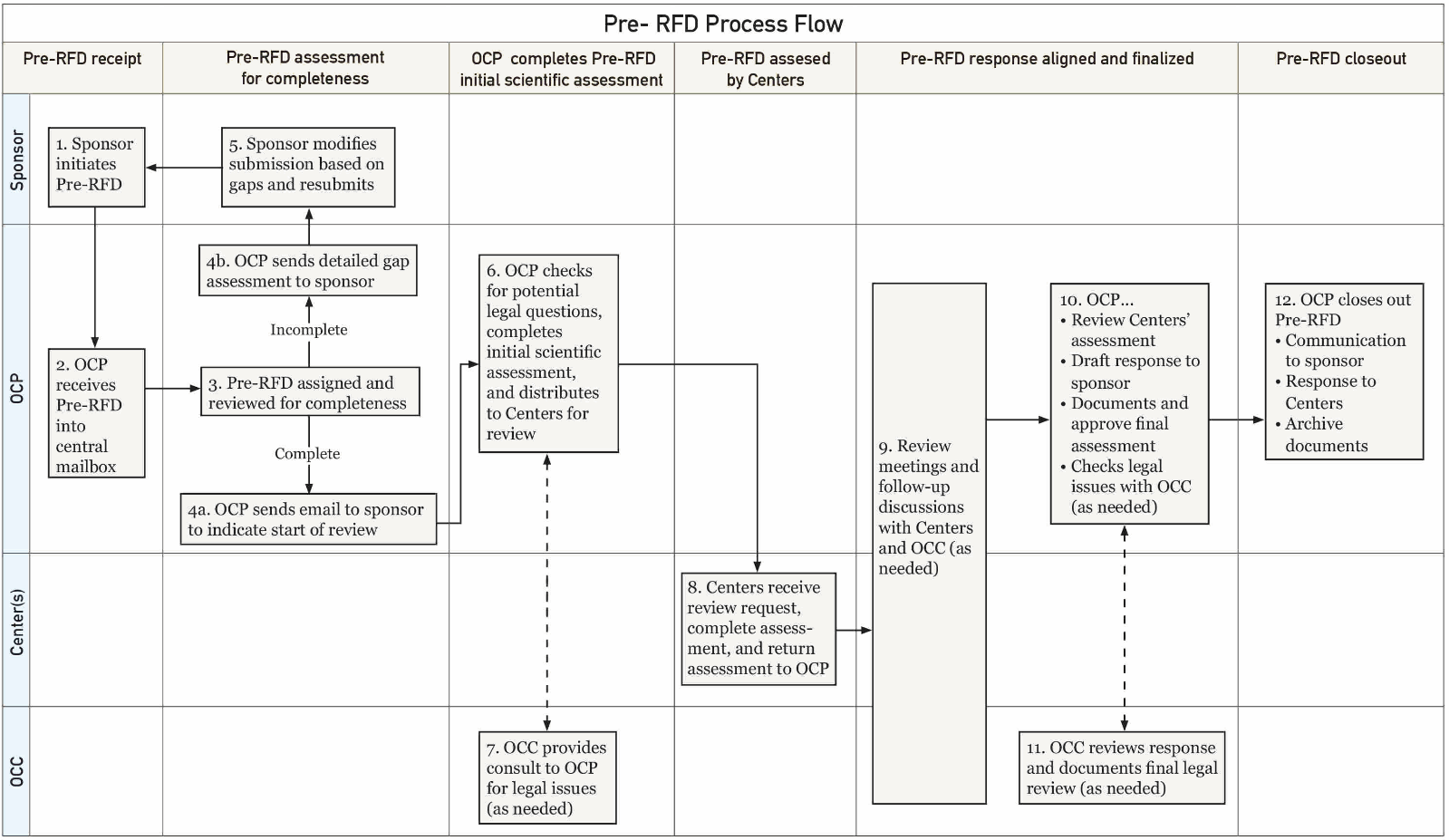
Now, FDA wants the same information included in a new process called Pre-Request for Designation (Pre-RFD), a less formal method to seek FDA feedback during a product's early stage of development, when the sponsor is contemplating a specific configuration of the product.
Writing in the agency blog FDA Voice, Thinh Nguyen, Director of OCP, and Rachel E. Sherman, M.D., M.P.H., FDA’s Associate Deputy Commissioner for Medical Products and Tobacco, detailed three key advantages of the Pre-RFD process.
- Sponsors are not required to provide a recommendation for classification and assignment of their product along with a corresponding rationale (e.g., bench studies, clinical studies) for that recommendation.
- Sponsors are not required to discuss the classification of currently marketed products that they believe to be similar to their product.
- Sponsors can receive preliminary feedback and information from the Agency that is derived from a structured and efficient process. The feedback will ultimately help lead to better decision-making and development of products for the sponsors.
The two officials wrote that FDA is developing a draft guidance about the Pre-RFD process, as well as a list of product classifications for various types of products.
The Pre-RFD process is the latest effort by FDA to streamline regulation of combination products, which have suffered delays due to separate procedures run by different centers.
Earlier this month, FDA announced its new intercenter consult request (ICCR) process designed to enhance efficiency in the communication and consultative interactions between FDA centers handling combination product reviews.
Below is a flowchart for the new Pre-RFD process.