Osmotic Controlled Release Oral Drug Delivery: A Closer Look
Osmotic controlled release drug delivery is not often spoken about, but it is a useful oral drug formulation technology.
In the realm of pharmaceuticals, precise control over drug release is paramount for achieving optimal therapeutic outcomes. One technology that has revolutionized drug delivery is the osmotic controlled release drug delivery. This dosage form ensures a controlled and steady release of medication over an extended period, offering numerous benefits over traditional delivery methods. This article will delve into the mechanism of osmotic controlled release drug delivery, its applications, advantages, and future prospects.
Understanding Osmotic Controlled Release Drug Delivery
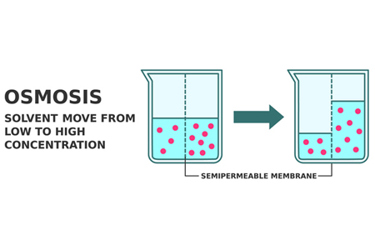
Osmotic controlled release drug delivery operates on the principle of osmosis, a natural process where solvent molecules move through a semi-permeable membrane from an area of lower solute concentration to an area of higher solute concentration.
The osmotic controlled release drug delivery consists of two key components: a drug reservoir and a semi-permeable membrane. The drug reservoir contains the medication in a concentrated form, along with an osmotic agent, usually a water-soluble salt. This creates a hypertonic solution inside the dosage.
When the osmotic controlled release dosage is introduced into the body, water from the surrounding environment permeates through the semi-permeable membrane into the drug reservoir. This influx of water increases the pressure within the dosage. As a result, the medication is forced through an orifice in a controlled manner.
Elements Of Osmotic Controlled Release Drug Delivery For Oral Dosage
- Semi-Permeable Membrane: The semi-permeable membrane plays an important role in the modulation of drug release from the osmotic drug delivery system. It should be stable in both the outer and inner environments of the dosage. The membrane should be rigid and inert and should maintain its dimensional integrity to provide a constant osmotic driving force during drug delivery. This membrane should be selectively permeable as the osmogen should not be lost by diffusion across the membrane during the passage of drugs and other ingredients present in the compartment. It should be biocompatible. It should exhibit sufficient water permeability so as to retain water flux rate in the desired range. The water vapor transmission rates can be used to estimate water flux rates. Any polymer that is permeable to water but impermeable to solute can be used as a coating material in osmotic dosages, e.g., cellulose esters like cellulose acetate and cellulose acetate butyrate. The semi-permeable membrane must possess sufficient wet strength (-105) and wet modulus to retain its dimensional integrity during the operational lifetime of the dosage.
- Plasticizers: Plasticizers are used in the coating membrane during the formulation of the osmotic system as they can change the viscoelastic behavior of the polymers, and these changes affect the permeability of the polymeric films. These changes can be modulated by using different types and amounts of plasticizers. Examples of some of the plasticizers are polyethylene glycols, ethylene glycol monoacetate, ethylene glycol diacetate, triethyl citrate, and diethyl tartrate or diacetin.
- Flux regulators: Delivery systems can be designed to regulate the permeability of the fluid by incorporating flux regulating agents in the layer. Hydrophilic substances such as polyethylene glycols (300 to 6,000 Da), polyhydric alcohols, polyalkylene glycols, and the like improve the flux, whereas hydrophobic materials such as phthalates substituted with an alkyl or alkoxy (e.g., diethyl phthalate or dimethoxy ethylphthalate) tend to decrease the flux. Insoluble salts or insoluble oxides, which are substantially water-impermeable materials, also can be used for this purpose.
- Wicking agents: A wicking agent is defined as a material with the ability to draw water into the porous network of a delivery device. A wicking agent is either swellable or non-swellable in nature. It is characterized by having the ability to undergo physisorption with water, which is a form of absorption in which the solvent molecules can loosely adhere to surfaces of the wicking agent via van der Waals interactions between the surface of the wicking agent and the adsorbed molecule. The function of the wicking agent is to carry water to surfaces inside the core of the tablet, thereby creating channels or a network of increased surface area. Materials that suitably act as wicking agents include colloidal silicon dioxide, kaolin, titanium dioxide, alumina, niacinamide, sodium lauryl sulphate (SLS), low molecular weight poly vinyl pyrrolidone (PVP), m-pyrol, bentonite, magnesium aluminum silicate, polyester, and polyethylene.
- Pore-forming agents: These agents are particularly used in the controlled release drug delivery developed for poorly water-soluble drugs and in the development of controlled porosity or multiparticulate osmotic drug delivery. These pore-forming agents cause the formation of a microporous membrane. The microporous wall may be formed in situ by the pore-former’s leaching during the operation of the system. The pore formers can be inorganic or organic and solid or liquid in nature. They include, for example, alkaline metal salts such as sodium chloride, sodium bromide, potassium chloride, potassium sulphate, potassium phosphate, etc.; alkaline earth metals such as calcium chloride and calcium nitrate; carbohydrates such as sucrose, glucose, fructose, mannose, lactose, sorbitol, mannitol, diols; and polyols such as poly hydric alcohols and polyvinyl pyrrolidone.
- Coating solvents: Solvents suitable for making polymeric solutions that are used for manufacturing the wall of the osmotic dosage include inert inorganic and organic solvents that do not adversely harm the core, wall, and other materials. The typical solvents include methylene chloride, acetone, methanol, ethanol, isopropyl alcohol, butyl alcohol, ethyl acetate, cyclohexane, water, etc. Mixtures of solvents such as acetone-methanol (80:20), acetone-ethanol (80:20), acetone-water (90:10), methylene chloride-methanol (79:21), and methylene chloride-methanol-water (75:22:3), etc., are generally used widely as coating solvents.
Advantages Of Osmotic Controlled Release Drug Delivery
Precise Drug Release: Osmotic drug delivery offers exceptional precision in drug release. The steady, controlled delivery minimizes fluctuations in drug concentration, ensuring a consistent therapeutic effect.
Reduced Dosage Frequency: Due to the sustained release nature of osmotic drug delivery, dosing frequency is significantly reduced. Patients often require only one administration every 24 hours, enhancing compliance and convenience.
Minimized Side Effects: By maintaining a constant drug concentration in the bloodstream, osmotic drug delivery can help mitigate potential side effects associated with rapid fluctuations in drug levels.
Improved Patient Comfort: Osmotic drug delivery eliminates the need for multiple daily doses, providing patients with a more comfortable and hassle-free treatment experience.
Enhanced Therapeutic Efficacy: The controlled release of medication allows for the maintenance of therapeutic levels in the body, leading to improved efficacy in managing chronic conditions.
Applications Of Osmotic Controlled Release Drug Delivery
Pain Management: Osmotic release drug delivery has found extensive use in the field of pain management. It is particularly beneficial for patients suffering from chronic pain conditions, providing sustained relief without the need for frequent dosing.
Cardiovascular Disorders: Medications for cardiovascular conditions, such as hypertension and angina, benefit from controlled release. Osmotic release drug delivery ensures that patients receive a consistent dose of medication throughout the day.
Psychiatric Medications: Conditions like schizophrenia and bipolar disorder often require long-term treatment with psychiatric medications. Osmotic release drug delivery can deliver these drugs steadily, minimizing the risk of relapse.
Hormone Replacement Therapy: Hormone therapies, including those for menopause or testosterone replacement, can be effectively administered using osmotic release drug delivery technology. This ensures a stable hormonal balance in the body.
Chronic Diseases: Osmotic release drug delivery is also used in the treatment of chronic diseases such as diabetes. They can provide a steady release of insulin, effectively managing blood glucose levels.
Examples (non-exhaustive list) of marketed products that use osmotic controlled release drug delivery mechanisms:
- Xeljanz XR
- Procardia XL
- Cardura XL
- Invega
- Adalat
- Ritalin
- Glucotrol XL
- Concerta
Future Prospects
As technology advances, the potential applications of osmotic drug delivery are expanding. Researchers are exploring ways to incorporate advanced materials and techniques to further enhance precision and versatility. Additionally, the development of personalized medicine may lead to customized osmotic drug delivery tailored to individual patient needs.
Osmotic controlled release drug delivery represents a significant leap forward in controlled release medication. Its precise mechanism, reduced dosage frequency, and improved therapeutic efficacy make it a cornerstone of modern pharmaceuticals. With ongoing research and innovation, the future holds even more promise for this remarkable technology, promising better treatment outcomes for patients around the world.
About The Author

Rajendran (Raj) Arunagiri has been in the pharma industry for a decade and has successfully developed and launched a new excipient. He is a co-author of technical articles and is an invited speaker at conferences focused on excipients and drug delivery. He specializes in the area of poorly soluble APIs and modified release. Arunagiri welcomes you to reach out to him for questions, comments, and collaboration ideas at raj.gceb@gmail.com.
