Waiting to inhale - news for the diabetes market
- Non-injected insulin will open up the lucrative type II diabetes market for insulin manufacturers; and
- Pfizer leads race to launch non-injected insulin.
Alternative routes of administration will rule
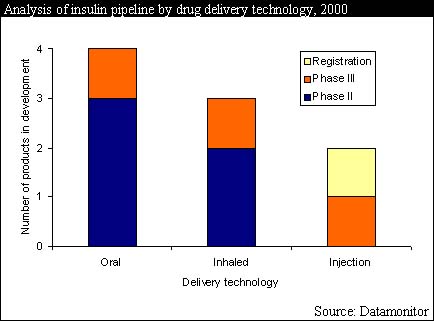
The prevalence of diabetes is rising worldwide, causing the diabetes drug market to expand rapidly each year. The leading class of drugs for treating diabetes is insulin, which is essential for type I diabetics who cannot produce insulin and is also often used to treat type II diabetics, who cannot produce the insulin quantity required. However, the inconvenience of injecting the drug has so far prevented insulin from realizing its full commercial potential in the far larger type II diabetic market.
With this in mind biotechnology companies and major insulin producing companies co-operating with other biotechnology companies are developing new, non-injected insulin products. This research has recently seen great progress and non-injected insulins will be readily available within two years, bringing great advantages to both the insulin manufacturer and the diabetic patient.
For the manufacturer of non-injected insulin, the financial rewards could be enormous, as it is expected that the majority of patients will switch when given the option. However, existing insulin users may only be the tip of the iceberg for the first non-injected insulin. Type II diabetics can often be well treated with insulin, however many do not use this therapy due to the requirement to inject. The launch of non-injected insulin will remove this barrier and open up the far larger type II diabetes market for insulin manufacturers.
The benefit to the patient will be twofold. Firstly the patients lifestyle will be greatly improved by not having to inject as frequently. Secondly, if the patient suffers type II diabetes the progression of the disease could be slowed through increased compliance with drug therapy.
Datamonitor Analyst Chris Donnellan comments, "The launch of inhaled insulin will not only see diabetics abandoning their syringes, but could also tempt some non-insulin users to change their ways."

Pfizer leading the race
Of the companies involved in the race to launch the first non-injected insulin product Pfizer has the lead. The collaboration of Pfizer with Inhale Therapeutic Systems and Avnet's has produced an inhaled insulin product that is currently undergoing phase III trials and is expected to be launched in 2002.
This will be the first non-injected insulin on the market and should therefore reap the rewards of first to market advantage. There is, however, still some doubt in the minds of doctors over the efficacy and safety of non-injected insulin products as a whole. However, Pfizer recently presented the results of long-term trials at the prestigious American Diabetes Association's 60th Scientific Sessions in San Antonio, and these data showed that long term use of Inhaled Insulin controlled the disease and was safe for the user.
Other companies involved in the production of non-injected insulin products include Novo Nordisk, Nobex, Generex and Cortecs, and the pressure will be on these companies to launch their products rapidly to prevent Pfizer gaining a stronghold on the market and seriously eroding their market shares. With the number of non-injected products set to rise during this decade the end could be in sight for all injected insulins.
According to Datamonitor Analyst Chris Donnellan, the current diabetes market will be revolutionized by Pfizer's inhaled insulin drug. "The structure of the insulin market may never be the same again," Donnellan said.
Drugs of Tomorrow 2000: Diabetes is available from Datamonitor, priced at $3,995.
For more information, contact Elisabeth Overend-Freeman of Datamonitor at 212-686-7400, ext 765, or efreeman@datamonitor.com.
Edited by Angelo DePalma
Managing Editor, Pharmaceutical Online and Drug Discovery Online
