ARTICLES BY RAJENDRAN (RAJ) ARUNAGIRI
-
Avoiding Alcohol-Induced Dose Dumping In Oral Drug Formulation8/21/2025
The FDA has become more vigilant about alcohol-induced dose dumping in extended-release oral dosages. Such dumping can result in anything from reduced therapeutic effects to dangerous toxicity. Let's take a closer look.
-
We Must Rethink The Solvents We Use For Peptide Synthesis4/21/2025
While the effectiveness of GLP-1 in weight loss is good news, the use of organic solvents in the GLP-1 synthesis and purification process is a growing pain that needs to be addressed.
-
Enteric Coatings For Oral Solid Dosages: A Short Guide11/19/2024
Enteric coatings play a pivotal role for drugs/APIs that need to withstand the harsh conditions of the stomach in order to dissolve in the intestine. This article shares an overview, commonly used excipients, and more.
-
The EU's Ban On TiO2 In Food May Impact Pharma. Here's What You Should Know.4/18/2024
Titanium dioxide (TiO2) is used as a food additive and OSD pharmaceutical excipient. In 2020, the European Food Safety Authority noted data gaps regarding particle size, which can affect its toxicological properties, so the European Commission banned it as a food additive. Here's how this could impact pharma.
-
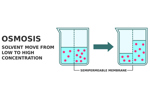
Osmotic Controlled Release Oral Drug Delivery: A Closer Look2/2/2024
Osmotic controlled release drug delivery is not often spoken about, but it is a useful oral drug formulation technology. This article shares the mechanisms of the elements of oral dosage, including the semi-permeable membrane, pore-forming agents, and more, as well as advantages and applications of this type of drug delivery.
-
4 Strategies To Formulate Poorly Soluble APIs12/18/2023
One of the toughest challenges facing small molecule drug development today is poor solubility of the API. No single technique has universal application, so several strategies exist. This article examines four notable strategies.
-
4 Tips To Leverage Your Ingredient Supplier For Faster Formulation8/3/2023
Speed to formulation plays a critical role in recouping the investment for a new drug. Many ingredient suppliers have an experienced analytical group and material scientist group, and drug developers should reach out and leverage that expertise with these four strategies.
-
3 Action Items For The US Bio/Pharma Industry To Mitigate Supply Vulnerabilities6/12/2023
The United States’ reliance on foreign manufacturers of API has been a known fact for several years. We can mitigate our supply vulnerabilities, but bio/pharma companies and the FDA both have roles to fill in this need.
-
Addressing 3 Challenges Of Developing Or Using A New Excipient5/8/2023
As the world discovers new molecular entities, new solutions for and innovation of excipients are more critical than ever. However, they are often overlooked. Let’s look at how to address three key challenges: risk aversion, an inconsistent feedback loop between pharma companies and excipient suppliers, and the current lack of regulatory body-backed innovation programs.