Accelerated patient recruitment reduces clinical trial time by 25%
By Frank Kilpatrick
Healthcare Communications Group
Patient recruitment continues as the major challenge to the timely completion of clinical research trials. Recruitment pitfalls include the growing number of competitive clinical research programs, the public's generally low awareness of and participation in trials, the need for proactive patient recruitment budgeting, and patient processing inefficiency. Because of these factors, 80% of all clinical studies are not completed on time.
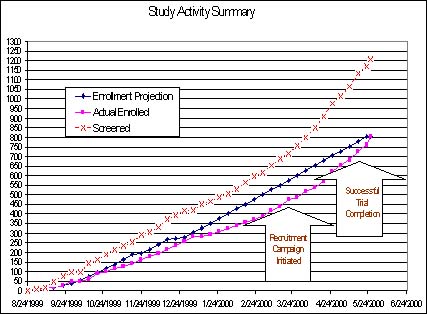
However, an innovative patient recruitment program that resulted in the on-time completion of a Major Depressive Disorder (MDD) study documents the value of professional enrollment techniques in solving recruitment challenges. This initiative combined the efforts of Healthcare Communications Group (HCC; Los Angeles) and a mid-sized contract research organization (Cato Research Ltd.) for a major pharmaceutical company's Phase IIB trial. It was implemented to remediate the declining accrual rate that began in late 1999 due to the exhaustion of PI internal patient databases and resulting drop in overall enrollment for this study. As the accompanying graph shows, the patient promotional campaign improved the clients' declining enrollment trend line and resulted in successfully recruiting the final group of subjects.
HCC's integrated recruitment program included television, radio, and newspaper ads, direct mail, and Internet promotion. Field-based initiatives also included PRN on-site personnel who assisted Principal Investigators' administrative teams by providing both management systems and personnel to focus attention on prospective patient contact and confirmation—along with working intensively with both Sponsor and contracted CRO personnel. Additionally, Healthcare deployed an Extranet Web Data Page to track media and site performance data in real time to facilitate mid-course tactical modifications.
Healthcare's recruitment effectiveness enabled the trial's sponsor to accelerate its compound's development within a crowded marketplace. Particularly validating was the comment of Cato Research Ltd. Senior Vice President-Research and Development Walker A. Long, who stated: "HCC's efforts gained the client $180 Million in additional product sales. We have become believers: Next time we will build in the patient recruitment program from the beginning."
The trend of professionalized, centralized clinical trial patient recruitment is rapidly becoming the gold standard in our industry.
 The cost of new drug compound development delay is frequently estimated as "exceeding $1 million per day." However, Healthcare President Frank S. Kilpatrick, analyzing CenterWatch statistics, reports: "For those pivotal, high-profile products with greater than average sales potential, the delay cost can frequently be a multiple of this figure. Increasingly, professional patient recruitment programs conducted by knowledgeable, experienced firms have become the standard for progressive pharmaceutical companies seeking proven solutions to the ongoing challenge of completing clinical trials on time. This represents an essential competitive advantage to their drug commercialization processes."
The cost of new drug compound development delay is frequently estimated as "exceeding $1 million per day." However, Healthcare President Frank S. Kilpatrick, analyzing CenterWatch statistics, reports: "For those pivotal, high-profile products with greater than average sales potential, the delay cost can frequently be a multiple of this figure. Increasingly, professional patient recruitment programs conducted by knowledgeable, experienced firms have become the standard for progressive pharmaceutical companies seeking proven solutions to the ongoing challenge of completing clinical trials on time. This represents an essential competitive advantage to their drug commercialization processes."
For more information: Jeffrey Rudolph, Director of Marketing and Business Development, Healthcare Communications Group, 880 Apollo St., Suite 347, El Segundo, CA 90245. Tel: 310-606-5700. Fax: 310-606-5705.
Edited by Angelo DePalma
Managing Editor, Drug Discovery Online and Pharmaceutical Online
