Achieving Integrated Quality Through A Continued Process Verification Program
By Peiyi Ko, Ph.D., founder and consultant, KoCreation Design LLC

To improve is to change. Continual improvement and technology adoption involve changes that are also important investments to stay compliant and keep up with regulatory commitments for the biotech/pharmaceutical industry.
The continued process verification (CPV) program is not only required for companies but also is a good investment in product quality and setting the foundation for continuous improvement. In part one of this two-part series, I’ll discuss the key elements and life cycle stage decisions of a CPV system and provide pointers for implementation. In the second part, I will look at how CPV signals and responses inform decisions for ongoing quality assurance, an example of important data-driven decisions that also support change management. I will also use a research paper on intelligent data analysis of sensor measurements to illustrate how an ontology framework can help with standardized automatic data collection, analysis, and alerts.
The main objectives of integrated quality are to increase process understanding, establish continuous quality monitoring and feedback, perform process analysis, and continuously improve. As it is always a journey to reach the ideal of integrated quality, I will discuss important aspects of change management (i.e., IT, business, and organization) that can be applied in any situation. It is my hope that concepts covered here can help you identify gaps and opportunities relevant to your organizations.
Regulatory Framework Overview
The FDA 2004 PAT (process analytical technology) framework and ICH guidance set out in Q8 through Q12 collectively highlight the keys to facilitating innovation in pharmaceutical development, manufacturing, and quality assurance and risk-based regulatory decisions by industry and the FDA with enhanced process understanding, whether through prior knowledge, process development, or manufacturing experience. Having an ongoing process monitoring program helps companies not only to be GMP compliant but also to manage quality risks effectively. The PAT framework guidance suggests companies look at it through two angles: (1) as a set of scientific principles and tools supporting innovation, arguably toward pharma 4.0 and (2) as a strategy for regulatory implementation that will accommodate innovation. This perspective is also applicable to the later ICH guidances. They help explain the reasoning behind integrated quality and help companies think about how to approach it, starting with development and manufacturing.
Continued Process Verification 101
cGMP requires companies to have a system that provides continuous quality monitoring and feedback from process analysis for continuous improvement. It can involve manual processes, which are error prone, or use software tools as part of a system that runs solely within the product owner’s facility or that is shared with the suppliers and/or CMOs. It can be viewed as a complex socio-technical system that involves both people and technologies working in a coordinated fashion to complete the tasks. Instead of a typical functional view, the work domain analysis from the cognitive work analysis is used to present a very simplified abstract-hierarchy of the system. For the scope given, a socio-technical system can be represented in multiple hierarchical levels, from broad/abstract to specific/concrete.
Purpose states the “why”: the purpose of the work system and the external constraints on its operation. As stage 3 of the process validation hierarchy, CPV is a formal means by which a commercial manufacturing process is monitored to ensure product quality. The purpose of a CPV system is to assure the commercial manufacturing remains in a state of control. Here, we also care about the external constraints, such as regulations, ICH guidances, and industry standards, that shape the strategic requirements of the program. These also affect the execution of the items at the lower levels and therefore need to be referenced.
Values and priority measures: the criteria that the work system use for measuring its progress toward the functional purposes. One of the priorities of a CPV program is to define signals that require responses and take appropriate actions, such as risk-based validation. For priorities, we are concerned with the criteria that would be used to determine success; for example, if the software system has a prevalidated platform offered by the vendor, that can be configured without revalidation or at least can reduce the validation burden to a customized, fit-for-purpose validation module.
Purpose-related functions: the general functions of the work system that are necessary for achieving the functional purposes. After understanding the purpose and priorities of the CPV program, we can then look into more detailed functional collaboration. Some typical groups that are involved in CPV are: management, development, validation/quality function, ops/process sciences/manufacturing science and technology, quality control, quality engineering, mathematical sciences/non-clinical statistics, and quality assurance.1 We can dedicate a role to any hardware/software that handles a process that used to be done by humans.
Below is an example analysis to the Function level (with the scope/boundary of a CPV program):
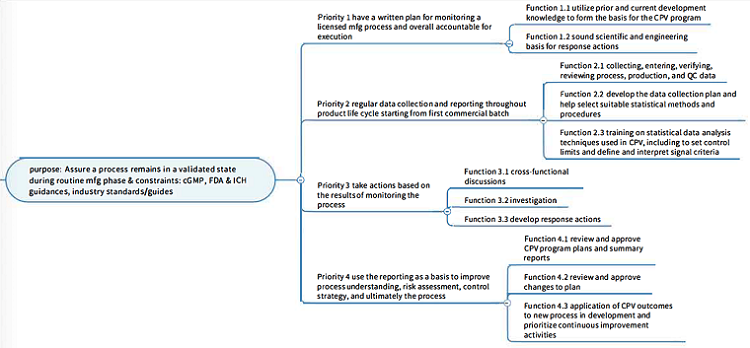
Object-related processes: The functional capabilities and limitations of physical objects and technologies in the work system that enable the purpose-related functions. There are many governing procedures for the tasks that each group has, and some require coordination across functions and may involve multiple decision points.
Physical objects and technologies in the work system that afford the object-related processes. Same philosophy as before: A knowledge base can be most beneficial when it consists of scientific understanding of the relevant multi-factorial relationships (e.g., between formulation, process, and quality attributes) as well as a means to evaluate the applicability of this knowledge in different scenarios (i.e., generalization). Today's information technology infrastructure makes the development and maintenance of this knowledge base more feasible. Approaches and information technology systems that support knowledge acquisition from such databases are valuable for the manufacturers and can also facilitate scientific communication with the agency.
After the purpose, the levels roughly correspond to information on product, people, process, and resources/materials. This analysis can be applied to a different defined scope depending on the resolution needed for a particular operational purpose. Next, let’s look at what’s expected of a CPV system, whether the tasks are done by humans and/or machines.
CPV Life Cycle Stage Decisions And Data
Based on the FDA’s process validation (PV) guidance, there are three stages, of which CPV is the third. PV stage 1, process design, and PV stage 2, process qualification, constitute the prework for CPV and signals within CPV can lead to responses that return to earlier process validation stages 1 or 2.2 The CPV program is often overlooked, but it is a good investment in product quality and the foundation for continuous improvements that take place over years of a product’s life cycle. Using informatics or IT systems to collect and analyze data not only helps to reduce chances of human errors that impose data integrity risks, if designed well and functions are validated, it also reduces the time needed to collect and process data to support risk-based decisions. It is recommended that users think about the informatics from the perspective of a journey of the data, including its touch points, processing, and consumption. Ultimately, the goal is for people to have the quality data and information they need to evaluate and respond to unsatisfactory outcomes and to enable opportunities for improvements.
For new products, the control strategy identified during development (i.e., stage 1, PV process design) is the product of process understanding and quality risk management (QRM). Further sampling and testing then take place in stage 2, PV qualification, to determine if, based on the intra- and inter-batch variabilities, the release testing and monitoring of the parameters and attributes can be reduced to routine CPV levels.3 Several resources within a company can help construct a CPV program: descriptions of process, control strategy for raw materials, process parameters and quality attributes, master plan, guideline and templates, SOPs for program and statistical methods, risk assessments, periodic CPV reports, and annual product reviews (APRs). As CPV goes on, periodic CPV reports and APRs, along with new regulations or market responses, will inform a continual improvement plan that can include updates on control strategy (CS), the CPV plan itself, and process changes (e.g., equipment, raw materials, and yield improvement).2
For existing/legacy products that don’t have CPV programs in place, process controls still need to be shown sufficient to provide customers with products that comply with specifications. Since there may not be critical quality attributes/critical process parameters (CQAs/CPPs) defined in the earlier submissions, criticality/risk assessments should be performed from past process performance data (e.g., in APR and/or batches before the formal stage 3) to define these. After determining the high-level strategy and gaps for remediation across product portfolios, start the product-specific evaluation by checking whether legacy release criteria and CQAs can be evaluated against process performance and variations. If not, establish a CPV program to achieve this goal.3
First, check whether process capability is weak or, if data is insufficient, collect more data through product capability studies or with enhanced CPV protocols as needed. Second, if the process is determined to be incapable, return to stage 1 process design to reevaluate CQAs/CPPs and find out which process steps and equipment add to variations. Third, if data is acceptable, go one level up to see if the monitoring/sampling approach provides a true view of critical process step capabilities and controls and confirm whether the current process represents what is filed. This can be the place to discuss regulatory impact and effect on the control strategy of the process change(s). Last, but not least, only when CQA data demonstrates effective process controls does it signal that it’s entering the stage of routine monitoring (i.e., late stage CPV).
In part two of this series, I will cover how CPV signals and responses inform decisions for ongoing quality assurance, as well as a research paper on intelligent data analysis of sensor measurements to illustrate how an ontology framework can help with standardized automatic data collection, analysis, and alerts.
Note: This article was based, in part, on a workshop presentation given by the author at the ISPE Annual Conference and Expo Oct. 17, 2019 (https://ispe.org/pharmaceutical-engineering/ispeak/pharma-quality-systems-regulatory-top-priorities-hot-topics).
References:
- BioPhorum Operations Group, Continued Process Verification: An Industry Position Paper With Example Plan (2014).
- M. Boyer et al, “A Roadmap for the Implementation of Continued Process Verification”, PDA J Pharm Sci and Tech (2016).
- ISPE, Good Practice Guide: Practical Implementation of the Lifecycle Approach to Process Validation (2019).
About The Author:
 Peiyi Ko, Ph.D., CHFP, founder and principal consultant of KoCreation Design LLC, strives to help companies and teams set the foundation for innovative continuous improvements in operations and work environment. Since 2017, she has collaborated with industry professionals and written articles for Life Science Connect to research and promote integrated quality and adoption of technology by the life science industry. She has executed and managed projects, presented at conferences and university classes, as well as led workshops in various fields. Her approach to decision analysis, training design, and project communication is inspired by human-system interaction research and human-centered design. She obtained her Ph.D. from the University of California, Berkeley, where she also completed the Engineering, Business, and Sustainability certificate and the Management of Technology certificate programs in 2011. You can reach her at info@kocreationdesign.com.
Peiyi Ko, Ph.D., CHFP, founder and principal consultant of KoCreation Design LLC, strives to help companies and teams set the foundation for innovative continuous improvements in operations and work environment. Since 2017, she has collaborated with industry professionals and written articles for Life Science Connect to research and promote integrated quality and adoption of technology by the life science industry. She has executed and managed projects, presented at conferences and university classes, as well as led workshops in various fields. Her approach to decision analysis, training design, and project communication is inspired by human-system interaction research and human-centered design. She obtained her Ph.D. from the University of California, Berkeley, where she also completed the Engineering, Business, and Sustainability certificate and the Management of Technology certificate programs in 2011. You can reach her at info@kocreationdesign.com.
