Addressing Challenges In Automated Visual Inspection Of Lyophilized Vials
By Sandeep Desai, independent subject matter expert

Lyophilization stabilizes biologics, vaccines, and injectables by removing water under vacuum, forming a porous cake. Regulatory agencies mandate 100% visual inspection of lyo vials to detect critical defects like cracks, melt-back, and particulate matter.1 Manual inspection, however, is subjective and inefficient, with human error rates exceeding 5%.2 Automated systems address this but struggle with the inherent variability of lyo cake structures and stringent GMP requirements.
This paper examines the technical challenges in automated inspection and evaluates modern solutions, including AI-driven defect classification and high-resolution imaging. The analysis aligns with USP <790> standards for visible particulates matter and ICH Q9 risk management principles.
Lyophilization involves three phases: freezing (-40°C to -50°C), primary drying (sublimation at 0.1–0.3 mbar), and secondary drying (residual moisture removal). Common defects include:
- Collapse: Loss of porosity due to insufficient glass transition temperature (Tg).
- Cracks: Mechanical stress during drying or stoppering.
- Particulate matter: Foreign matter (e.g., glass fragments, fibers) exceeding USP <788> limits.
Automated systems use monochromatic or RGB cameras with structured lighting (e.g., LED arrays at 30° to 60° angles) to capture vial images. Rule-based algorithms historically classified defects using edge detection and pixel intensity thresholds. However, these methods fail to adapt to batch-to-batch variability in cake appearance.
Challenges in Automated Visual Inspection
The automated visual inspection of lyophilized vials is beset by a myriad of challenges that stem from the inherent variability of the lyophilized cake structure, limitations of imaging hardware, and the rigidity of regulatory frameworks. One significant technical challenge arises from the heterogeneity of lyo cake morphology. Variations in freezing rates, primary drying conditions, and secondary drying protocols result in diverse physical characteristics such as differences in porosity, crystallinity, and surface texture.4
These differences profoundly affect the contrast and edge characteristics in images, complicating the task of reliably segmenting the cake region from the background and differentiating genuine defects from acceptable variations. The subtle gradations between normal morphological variations and early-stage defects necessitate extremely high-resolution imaging and sophisticated processing algorithms that can adapt to batch-to-batch variability without sacrificing sensitivity.5
In addition, the dynamic range and spectral sensitivity of current imaging sensors often prove insufficient for capturing the full detail of the lyophilized product, particularly when dealing with translucent or low-contrast features.3,6 The performance of traditional monochromatic or RGB cameras is further compromised by the variability in illumination conditions; even minor deviations in structured lighting angles or intensities can introduce shadows or reflections that mimic defects.
Adaptive lighting systems, while beneficial, require precise calibration and are highly sensitive to environmental fluctuations, thereby increasing the complexity of system integration and maintenance. Moreover, the reliance on rule-based algorithms, which depend on predefined thresholds and edge detection methods, results in high false-positive rates when confronted with irregular textures or unexpected light scattering phenomena.5,7
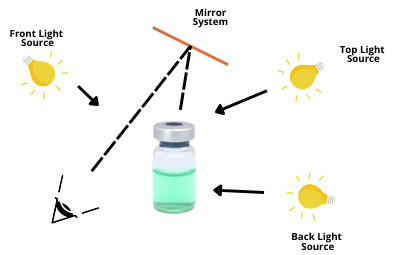
Figure 1: Lyophilized vial inspection via different light sources
The incorporation of advanced machine learning techniques, such as convolutional neural networks (CNNs), presents its own set of challenges. The development of reliable models is contingent upon the availability of large, well-annotated datasets that capture the full spectrum of normal and abnormal variations in lyophilized cakes. Given the stringent quality requirements and the relative infrequency of certain defect types, acquiring a statistically significant number of defective samples is inherently difficult. This scarcity can lead to overfitting and poor generalization in machine learning models, thereby undermining their reliability in production settings.8
Furthermore, the computational overhead associated with training and deploying deep learning models in a real-time manufacturing environment necessitates significant hardware investments and rigorous system validation to ensure compliance with regulatory standards.
Finally, the integration of these advanced inspection systems into existing manufacturing processes is hampered by strict regulatory mandates such as USP <790>, USP <788>, and EU Annex 1.8 These regulations impose rigorous validation requirements that necessitate detailed documentation and extensive performance testing to show that the automated systems do not compromise product quality or patient safety. The challenge is compounded by the need for the systems to operate under diverse production conditions, requiring flexible yet reproducible algorithms that can be seamlessly validated within the established regulatory framework.8
This shows that while automated visual inspection holds considerable promise for enhancing product quality and operational efficiency, its implementation is fraught with challenges that demand meticulous attention to system design, data quality, and regulatory compliance.
Technological Solutions for Improved Accuracy
The technological evolution in visual inspection systems for lyophilized vials has catalyzed the integration of diverse, high-precision methodologies that address the complex challenges inherent to pharmaceutical manufacturing.
Hyperspectral Imaging for Subtle Deviations
Hyperspectral imaging (HSI) represents a significant advancement in this domain. By capturing data across a continuous spectrum from 400 to 1000 nm, HSI systems enable the identification of subtle chemical deviations within the lyophilized cake, such as early oxidation or sucrose degradation, that may not be perceptible through conventional imaging techniques.9
This modality effectively enhances the precision of defect identification while providing an avenue for non-destructive chemical analysis, which is essential for maintaining the integrity of sensitive biologics.
Deep Learning Models as a Prerequisite to AI Defect Detection
Deep learning, particularly through the application of CNNs, also has become a building block for modern visual inspection solutions. Pretrained architectures such as ResNet-50 have been successfully fine-tuned using extensive image datasets comprising over 50,000 vial images, leading to a marked reduction in false negatives by approximately 30%.10
The use of transfer learning further refines these models, enabling them to adapt to new product lines with relatively small training sets of fewer than 1,000 images. This adaptability is critical in the pharmaceutical sector where product variability is the norm and rapid model recalibration is required to ensure consistent performance across diverse batches. The capacity of CNNs to automatically extract intricate features and classify subtle variations in lyo cake morphology shows their potential to revolutionize defect detection protocols in lyophilized vials.
High-Res and 3D Imaging
The incorporation of 3D laser scanning techniques further augments the analytical capabilities of inspection systems. Laser triangulation methods facilitate precise measurement of cake height deviations with a resolution of ±0.1 mm, effectively capturing microstructural anomalies such as cracks that may be indiscernible in 2D imaging frameworks.5,7,11
Systems such as Siemens SIMATIC PVS have reported repeatability metrics as high as 99.5% in the analysis of vial curvature, which is critical for ensuring uniformity in physical parameters that influence both product efficacy and stability. The transition from traditional 2D to 3D analysis permits a more reliable characterization of the lyophilized product, bridging the gap between surface-level inspection and comprehensive structural evaluation.
Different Lighting for Full-Spectrum Analysis
Dynamic lighting systems constitute another solution by addressing the challenges posed by variable illumination conditions. The implementation of adaptive LED arrays, which adjust both intensity and angular disposition in response to the optical properties of the lyophilized cake, mitigates the occurrence of glare and shadow artifacts that can compromise image quality. Optimization software, such as Synopsys’ LightTools, is employed to fine-tune these lighting parameters in real time, reducing glare-induced errors by an estimated 40%.5
This dynamic lighting approach ensures that the inspection system consistently captures high-fidelity images, thereby enhancing the accuracy of subsequent image processing and defect classification algorithms.7
Artificial Intelligence-Driven Defect Detection for Lyophilized Vials
The integration of artificial intelligence (AI) frameworks into the defect detection process for lyophilized vials has markedly enhanced the accuracy and reliability of visual inspections. Advanced machine learning models, particularly CNNs, have been developed to automatically classify and identify subtle defects that traditional rule-based systems frequently overlook.13
Once trained on large datasets that include a wide range of normal and aberrant lyo cake morphologies, AI models have expressed the capacity to distinguish between acceptable variations and true defects with high precision. The deep learning models discussed above can be a prerequisite for this, serving as the primary database for AI models.14,15 For instance, studies have reported that CNN-based models can achieve near-perfect precision and recall on critical defects, significantly reducing both false positives and false negatives compared to conventional inspection methods.
AI defect detection systems benefit from the ability to learn and adapt continuously, thereby mitigating the risks associated with operator fatigue and subjective judgment. This dynamic learning capability allows the models to update their predictive algorithms as new data become available, which is particularly valuable given the inherent variability in lyophilized cake structures caused by fluctuations in process parameters. The use of transfer learning further enhances these systems by enabling the rapid adaptation of pre-trained models to new product lines with minimal additional data, thus streamlining the development process and maintaining high detection accuracy even when labeled datasets are limited.15
Moreover, the integration of AI-based defect detection with complementary technological solutions such as hyperspectral imaging and dynamic lighting systems creates a synergistic effect. Hyperspectral imaging provides detailed spectral data that can reveal chemical deviations in the lyophilized product, while adaptive lighting systems ensure consistent image quality by compensating for variations in illumination. Together, these enhancements reduce the risk of misclassification and improve the overall reliability of the inspection process. As a result, pharmaceutical manufacturers can achieve a more consistent product quality and lower the incidence of costly re-inspections, thereby ensuring compliance with stringent regulatory standards and safeguarding patient safety.16
Implementation and Validation of Advanced Visual Inspection Systems
Data acquisition protocols must be carefully designed to ensure that the training datasets encompass the full spectrum of variability in lyophilized cake morphology, including rare defect manifestations. In practice, this involves high-resolution imaging under controlled lighting conditions and continuous collection of eject images from automated vision inspection (AVI) systems. The accumulation of such extensive datasets is paramount for training CNN models that can discern between acceptable product variations and genuine defects with high precision.8,16
Integration of the AI framework into existing manufacturing environments requires a systematic approach that aligns with current regulatory guidelines such as USP <790>, USP <788>, and EU Annex 1. Validation protocols must address the reproducibility of the AI system’s performance over extended operational periods, including sensitivity, specificity, and overall defect detection rates.8,15
Quantitative metrics such as false reject and false accept rates are calculated through iterative testing phases that simulate real-world manufacturing fluctuations. Pilot implementations on dedicated production lines facilitate controlled assessments wherein the AI system’s predictions are cross-validated against manual inspections and traditional rule-based algorithms. This comparative analysis is essential to verify that the AI-driven systems surpass the performance of legacy systems, thereby ensuring a reduction in re-inspection rates and improved throughput.
Furthermore, the implementation phase involves integration of hyperspectral imaging and dynamic lighting systems, which are calibrated in tandem with the AI modules to optimize defect detection accuracy. This integration is achieved through the development of middleware that synchronizes image capture, processing, and decision-making modules in real time. The AI system’s ability to update its predictive algorithms through transfer learning is critically evaluated during validation trials, allowing for rapid adaptation to novel product variations or new defect types.
Advanced statistical methods and confidence interval analysis are employed to assess the consistency and reliability of the system across multiple batches and production shifts. Independent studies have corroborated the potential for AI-driven approaches to yield significant improvements in defect detection, with some reports indicating enhancements in accuracy of up to 99.9% and corresponding reductions in false rejection rates.
Conclusion
The integration of advanced imaging technologies and AI-driven defect detection systems in the visual inspection of lyophilized vials represents a transformative leap in pharmaceutical quality assurance. The research delineated herein has systematically addressed the inherent challenges of traditional inspection methods — ranging from the variability in lyophilized cake morphology to the limitations imposed by static imaging and heuristic-based algorithms — and has shown that a synergistic approach combining hyperspectral imaging, dynamic lighting, 3D laser scanning, and deep learning architectures can markedly enhance detection accuracy.
The rigorous implementation and validation of these systems under real-world manufacturing conditions have underscored their capacity to reduce false reject and accept rates, thereby minimizing re-inspection costs while ensuring compliance with stringent regulatory frameworks such as USP <790>, USP <788>, and EU Annex 1.
Furthermore, the utilization of CNNs augmented by transfer learning has proven critical in adapting to the extensive variability of lyophilized products. The continuous improvement capabilities inherent in AI frameworks enable these systems to refine their predictive accuracy as more data are accrued, ensuring sustained operational efficiency and reliability.
The convergence of these technological innovations supports a significant reduction in inspection errors while leading the way for enhanced PAT integration, creating a more responsive and data-driven manufacturing environment.
References
- J. G. Shabushnig, “Visual inspection of injectable products: More than sorting good from bad …,” Insight Pharma Consulting, LLC, Presentation, Sept. 2022. [Online]. Available: https://www.fda.gov/media/162175/download.
- F. Jameel, A. Alexeenko, A. Bhambhani, G. Sacha, T. Zhu, S. Tchessalov, L. Kumar, P. Sharma, E. Moussa, L. Iyer, R. Fang, J. Srinivasan, T. Tharp, J. Azzarella, P. Kazarin, and M. Jalal, "Recommended best practices for lyophilization validation-2021 Part I: Process design and modeling," AAPS PharmSciTech, vol. 22, no. 7, p. 221, Aug. 2021, doi: 10.1208/s12249-021-02086-8. [Erratum in: AAPS PharmSciTech, vol. 22, no. 8, p. 250, Oct. 2021].
- S. Ullrich, S. Seyferth, and G. Lee, "Measurement of shrinkage and cracking in lyophilized amorphous cakes. Part IV: Effects of freezing protocol," International Journal of Pharmaceutics, vol. 495, no. 1, pp. 52–57, 2015. [Online]. Available: https://api.semanticscholar.org/CorpusID:205332445
- K. Sookne, "Study: Automatic Inspection for Lyophilized Drug Products in Valor Vials," Healthcare Packaging, Aug. 12, 2020. [Online]. Available: https://www.healthcarepackaging.com/machinery/packaging-filling/article/21159812/study-automatic-inspection-for-lyophilized-drug-products-in-valor-vials.
- M. Gatto and W. Missaoui, "Lyophilization of Nanoparticles, Does It Really Work? Overview of the Current Status and Challenges," International Journal of Molecular Sciences, vol. 24, no. 18, p. 14041, Sep. 2023, doi: 10.3390/ijms241814041.
- R. Remmele, K. Sampathkumar, and W. Callahan, "Development of Stable Lyophilized Protein Drug Products," Curr. Pharm. Biotechnol., vol. 13, pp. 471–496, Jan. 30, 2012, doi: 10.2174/138920112799361990.
- K. A. Overhoff, K. P. Johnston, J. Tam, J. Engstrom, and R. O. Williams III, "Use of thin film freezing to enable drug delivery: a review," J. Drug Deliv. Sci. Technol., vol. 19, no. 2, pp. 89–98, 2009, doi: 10.1016/S1773-2247(09)50016-0.
- L. Alzubaidi et al., “Review of deep learning: concepts, CNN architectures, challenges, applications, future directions,” Journal of Big Data, vol. 8, no. 1, Mar. 2021, doi: 10.1186/s40537-021-00444-8.
- Gâvan, C. Bogdan, L. Rus, P. Rșteiu, I. Toma, M. Achim, and S. Iurian, "NIR spectroscopy coupled with multivariate data analysis in the prediction of the characteristics of mannitol lyophilized cakes," Rom. J. Pharm. Pract., vol. 14, no. 3, 2021.
- L. Alzubaidi et al., "Review of deep learning: concepts, CNN architectures, challenges, applications, future directions," Journal of Big Data, vol. 8, no. 53, Mar. 2021, doi: 10.1186/s40537-021-00444-8.
- K. Azlan, R. Omar, M. S. F. Hussin, M. I. H. Chua, and E. Chinniah, "Measurement accuracy assessment for laser triangulation 3D scanning machine," Int. J. Recent Technol. Eng. (IJRTE), vol. 8, pp. 2789–2793, Mar. 2020, doi: 10.35940/ijrte.F8394.038620.
- “SIMATIC PCS 7 – the distributed control system with proven performance,” siemens.com Global Website. https://www.siemens.com/global/en/products/automation/process-control/simatic-pcs-7.html
- Z. Li and C. Tsay, “WO2020081668A2 - Defect detection in lyophilized drug products with convolutional neural networks - Google Patents,” Oct. 19, 2018. https://patents.google.com/patent/WO2020081668A2/en
- “Vision Inspection using Machine Learning/Artificial Intelligence,” ISPE | International Society for Pharmaceutical Engineering, Feb. 13, 2025. https://ispe.org/pharmaceutical-engineering/november-december-2020/vision-inspection-using-machine
- Q. Herve, N. Ipek, J. Verwaeren, and T. De Beer, "A deep learning approach to perform defect classification of freeze-dried product," Int. J. Pharm., vol. 670, p. 125127, Feb. 2025, doi: 10.1016/j.ijpharm.2024.125127.
- P. Tran, Automated Visual Inspection of Lyophilized Products via Deep Learning and Autoencoders, M.S. thesis, 2021.
About The Author:
Sandeep Desai has 20+ years of experience in pharmaceutical engineering, specializing in HVAC systems, water systems, and manufacturing process equipment. With years of experience in facility design, qualification, and process optimization, he has implemented advanced HVAC solutions to maintain classified environments and ensure regulatory compliance. Adjacent experience lies in designing, installing, and optimizing purified water, WFI, and clean steam systems to meet stringent pharmaceutical standards. He has led modifications and enhancements in filling lines, lyophilizers, autoclaves, and sterilization systems to improve efficiency, reliability, and product integrity. In previous roles, he has worked with OSD and ointment facilities.
