Alba®: A Breakthrough Solution For Biologics
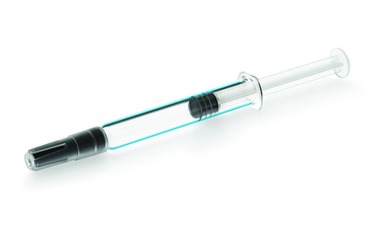
Medicines are highly sensitive to primary packaging components, which can affect compatibility with container closure systems. Discover Alba® syringes, designed for sensitive biologics, including highly concentrated drugs vulnerable to silicone interaction and ophthalmic medications.
De-risking the development of sensitive drugs.
Alba® EZ-fill® syringes represent the best-in-class solution for biologics and ophthalmic applications addressing the key requirements of protein-based drugs, and minimizing adsorption, aggregation and inorganic extractable interaction.
All Alba® EZ-fill® products have an internal coating, based on standard silicone oil, which is cross-linked to form a chemical bond with the glass surface. It ensures that particle levels - caused by silicone migration during container storage - are significantly lower than ones using standard siliconization technologies. Moreover, the internal treatment offers superior gliding performance, reducing the risk of functional issues when combined with auto-injectors.
Due to the excellent properties of the layer, Alba® platform mitigates packaging-related risks and enhances drug-interaction stability.
Available Formats:
Benefits:
- Particle reduction - Minimized subvisible particles versus conventional sprayed-on siliconized containers to alleviate potential drug-silicone interaction.
- Optimized break-loose and glide performance - Silicone lubricant layer integrity, optimized to withstand challenging formulations and aggressive conditions, helps conserve gliding performances throughout the drug shelf-life.
- Reduction risk of stalling and injection time variation - The low silicone-drug interaction helps to maintain the injection time and dose delivery with an auto-injector.
- Inorganic extractable mitigation - A barrier between the drug and the glass reduces the amount of inorganic extractables.
- Time stability - Alba® inner coating does not undergo critical variations after contact with the placebo solution over a period of 6 months (tested @40°C/75%RH).
Preferred Market Choice:
- Protein-based therapeutics
- Ophthalmic applications

