Alliance Pharmaceutical, Baxter to Collaborate on Oxygent
 Alliance Pharmaceutical Corp. (San Diego) and Baxter Healthcare Corp. (Deerfield, IL) have reached an agreement for manufacturing, selling, and distributing Alliance's Oxygent (perflubron emulsion) in the United States, Canada, and Europe. Oxygent, a chemical-based oxygen carrier, enhances oxygen delivery to tissues. Used with a procedure called augmented-acute normovolemic hemodilution (Augmented-ANH), Oxygent could eliminate or reduce the need for blood transfusion during surgery.
Alliance Pharmaceutical Corp. (San Diego) and Baxter Healthcare Corp. (Deerfield, IL) have reached an agreement for manufacturing, selling, and distributing Alliance's Oxygent (perflubron emulsion) in the United States, Canada, and Europe. Oxygent, a chemical-based oxygen carrier, enhances oxygen delivery to tissues. Used with a procedure called augmented-acute normovolemic hemodilution (Augmented-ANH), Oxygent could eliminate or reduce the need for blood transfusion during surgery.
Under the agreement, Baxter will purchase $20 million of Alliance convertible preferred stock later this month. For Baxter to maintain its rights to commercialize Oxygent, it must invest an additional $30 million over the next 18 months as clinical trials progress. Once Oxygent is launched, Alliance will also receive royalty income and an ongoing share in profits from sales.
Baxter's investment will support Alliance's late-stage clinical trials regulatory filings. Baxter will obtain an exclusive manufacturing, sales, and distribution license for Oxygent in the United States, Canada, and Europe, and will have the rights to co-develop Oxygent for other indications.
"As we considered potential partners for Oxygent we concluded that Baxter was the best possible company due to their recognized leadership in marketing surgical products," said Duane J. Roth, Alliance's chairman and CEO. "The Baxter division responsible for marketing the product focuses on anesthesiologists, the physicians most responsible for transfusion decisions. This makes them an ideal marketing partner for Oxygent."

Alliance manufacturing suite where perfluorinated hydrocarbon therapeutics are made.
"Oxygent complements our business strategy of expanding our proprietary anesthesia and critical care pharmaceuticals through the addition of currently marketed products and compounds in late-stage clinical development," said Jack McGinley, group vice president of Baxter. "Oxygent also complements other Baxter development programs."
Efforts to develop oxygen-carrying products have focused on one of two technologies: hemoglobin, which may be derived from various biologic sources (including recombinant technology), or synthetic perfluorinated organic compounds, the chemical class to which Oxygent belongs. Perfluorinated hydrocarbons are clear fluids that dissolve large amounts of respiratory gases such as oxygen and carbon dioxide, and can be formulated into stable intravenous emulsions. This emulsion is designed to increase the oxygen-carrying capacity of blood. Perfluorinated materials differ from hemoglobin-based oxygen-carrying solutions in that they are most effective at higher oxygen levels, making them ideal for situations where patients are already receiving elevated oxygen, such as during surgery. Perfluorinated hydrocarbons are also more efficient oxygen carriers than hemoglobin, delivering more than 90% of their dissolved oxygen to tissues—versus only about 20% for hemoglobin.
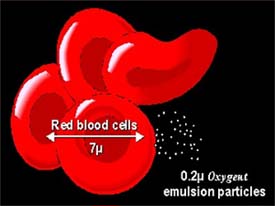
>Their small size relative to red blood cells allows Oxygent emulsion microspheres to permeate and saturate cells with oxygen.
Oxygent is being developed for use with the Augmented-ANH technique to reduce and/or eliminate the need for donor blood transfusion during surgeries of expected high blood loss. Augmented-ANH involves the collection of several pints of blood from the patient immediately before surgery. Oxygent is then be administered to replace the oxygen-carrying capacity of the collected blood. When surgery is over, the patient's blood that was removed and conserved during surgery would then be re-infused to achieve a safe red cell concentration for postoperative recovery. Augmented-ANH is thereby intended to minimize the need for donor blood transfusions during and after surgery. Minimizing blood transfusions are is highly desirable due to blood shortages, the reduced ability of red blood cells to transport oxygen effectively after extended storage, and to prevent transmission of blood-borne diseases.
Oxygent has been administered to more than 1,000 patients in clinical trials. A Phase 3 European study involving patients undergoing cancer, urologic, vascular, orthopedic, and other major surgeries is expected to complete enrollment next month. An additional Phase 3 study involving patients undergoing coronary artery bypass grafting is underway in the United States and Canada. Oxygent is the only oxygen-carrying therapeutic in Phase 3 whose studies were designed to support a label claim for use in patients undergoing both cardiac and non-cardiac surgical procedures.
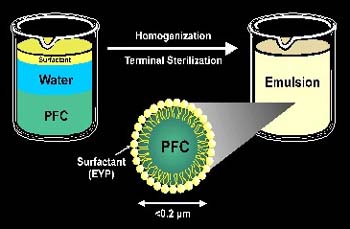
0.2-micrometer Oxygent microspheres are made by combining perfluorinated hydrocarbon, water, and surfactant.
Alliance Pharmaceutical Corp. develops therapeutic and diagnostic products based on its perfluorochemical and surfactant technologies. Alliance products are intended primarily for acute care situations, especially during surgery and other invasive procedures. In addition to Oxygent, Alliance is developing LiquiVent, an intrapulmonary liquid ventilation agent being evaluated in pivotal Phase 2-3 studies in North America and Europe for treating acute lung injury and acute respiratory distress syndrome. Alliance has filed a New Drug Application with the U.S. Food and Drug Administration for approval of Imagent, an ultrasound contrast agent being developed with Schering AG.
Baxter Healthcare Corp. is the principal U.S. subsidiary of Baxter International Inc., a global medical products and services company that provides therapies for people with life-threatening conditions. Baxter's products and services are marketed in more than 100 countries.
For more information: Gwen Rosenberg, Alliance Pharmaceutical Corp., 3040 Science Park Rd., San Diego, CA 92121. Tel: 858-410-5275. Fax: 858-410-5201.
