AMI Seal Integrity Test System: Blisters, Vials, Bottles

Optical emission spectroscopy, an innovative CCIT solution for the pharmaceutical industry.
With the AMI 1000, the products can be sampled directly from the production line and loaded in the test chamber without any specific conditioning. At the end of the test sequence, the result is clearly displayed and a PDF report is automatically generated at the batch closure. Full automation of the test cycle including loading/unloading of the samples can be easily implemented for in-line tests.

Dedicated to the pharmaceutical industry
AMI equipment has been qualified by leading pharmaceutical companies as in-process control (IPC) leak testing for blister packs. Our software is CFR21 part 111) compliant.
 Large detection range
Large detection range
Different detection methods can be combined in order to cover the complete detection range. Massive leak and fine leak tests are performed within a single test sequence, any additional gross leak test (e.g. blue dye ingress) can be omitted.
Deterministic test method
As no operator intervention is required, the measurement results are totally objective. High accuracy measurements can be achieved thanks to a calibration-validation sequence of the equipment based on certified calibrated leaks.
High sensitivity, high throughput
High sensitivity tests combined with high throughput enables trend analysis to early indicate production issues. In high sensitivity mode, O.E.S is able to detect 0.2 μm defect size on glass containers which corresponds to the sterility barrier defined as the MALL (Maximum Allowable Leakage Limit) in the USP2) <1207> guidelines.
1) Code of Federal Regulations by the United States Food and Drug Administration (FDA) 2) United State Pharmacopia
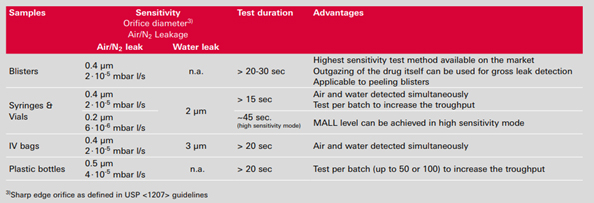
Packaging Examples

Blister packs

Glass vials

Plastic bottles
