Atrix Proves Safety and Effectiveness of Systemic Drug Delivery Technology
The Atrix study showed Atrigel to be safe and effective for systemic delivery of leuprolide acetate, a leutenizing hormone-releasing hormone agonist, over a 30-day period to suppress prostate cancer growth. Atrigel was originally developed for site-specific delivery of an antibiotic for treating periodontal disease.
"This confirms for the first time since its discovery that Atrigel works for the systemic delivery of pharmaceuticals in the body, and paves the way for its use in a broad range of therapeutic areas where timed release of pharmaceutical products is needed," said David Bethune, vice chairman and CEO of Atrix. With this discovery, Atrix becomes a significant—although not quite yet a major—player in the drug delivery arena. Adds Bethune, "We're one of only a few companies whose innovative drug delivery systems have demonstrated therapeutic results in humans."
According to Atrix, drug delivery is a $2 billion business that delivers a high return on investment. "Large pharmaceutical companies are always looking for new ways to improve and extend the utility of their existing product lines, and the Atrigel drug delivery system can provide both medical and economic benefits," Bethune said. "Atrigel will also make important new therapies possible and is expected to be especially valuable for the safe and effective administration of the expanding array of new chemical entities and biotechnology products that cannot be taken by mouth."
Atrigel
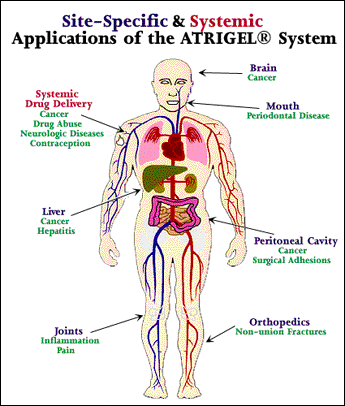
Atrigel polymeric drug delivery uses biodegradable polymers and biocompatible carriers incorporating the drug to be delivered. Although Atrigel can take the form of solutions, gels, pastes, and putties, the preferred applications are those in which liquid product is injected with a syringe and a small gauge needle.
When the delivery system interacts with tissue fluids, the polymer precipitates to form a solid implant in situ, which releases the drug in a controlled manner and subsequently biodegrades. The system can be designed for either local or systemic therapy. This biodegradable polymer system also has the potential to form biodegradable medical devices in situ. For some applications, the precipitated polymer without a drug may form a useful localized barrier function, such as guided tissue regeneration surgery and prevention of surgical adhesions. In addition to providing a physical barrier to protect the wound from the external environment, Atrigel can also be used to prevent infections and stimulate healing through incorporation of antibiotics and growth factors, respectively, in the polymer system.
Benefits
"Atrigel is not only easier to use by the medical profession and more acceptable to patients than other drug delivery systems, but is much simpler and less costly to manufacture, and offers many more options for therapeutic applications," said Richard Jackson, Atrix senior vice president of research and development. "It allows for the sustained release of virtually any pharmaceutical ranging from small molecules to complex peptides and proteins, and over times ranging from a few days to several months."
Unlike microcapsules or microspheres, which are usually injected intramuscularly, Atrigel can also be administered subcutaneously (under the skin), which is simpler and less painful, and can be easily retrieved if treatment needs to be terminated. It can be administered with a standard needle and syringe, and unlike many implants now used for long-acting drug delivery, it is bioabsorbable and does not require surgical removal. Atrigel sustained release technology allows for a variety of drug release profiles, providing predictable release kinetics over days to months.
Other benefits include:
- Wide compound applications, ranging from traditional small synthetic molecules to very large peptides and proteins.
- Can be injected or inserted as a solution, paste, gel or putty by means of ordinary cannulas and syringes, or through other appropriate means of placement.
- Biodegradable, thus not expected to require removal when the drug is depleted.
- Localized or systemic delivery.
- No patient compliance issues since the drug is implanted and released over time.
R&D
Now that Atrix knows Atrigel is safe and effective, it plans to use it to develop products for treating cancer, chronic pain and many other conditions that can benefit from a long-acting delivery system. In addition, the innovative system offers opportunities for substantial advances in the long-term treatment of mental illness and other chronic disorders where patient compliance is a major issue.
Atrix has two dental products on the brink of commercialization and is working with partners to evaluate a variety of Atrigel medical applications. Examples of the types of drugs delivered using Atrigel include anesthetics, antibiotics, antigens (vaccines), antimicrobials, antineoplastics, antipsychotics, cytokines, enzymes, growth factors, hormones, narcotic antagonists, and non-steroidal antiinflammatory drugs.
Primary target disease application for the Atrigel technology include orthopedic surgery, post-operative pain, and solid tumor cancers. Other promising areas for the potential use include osteomyelitis, dermal wounds and ulcers, hormone replacement therapy, chronic anemia, vaccination/immunization, heroin addiction and schizophrenia.
For more information: Richard Jackson, Sr. VP of R&D, Atrix Laboratories, Inc., 2579 Midpoint Drive, Fort Collins, CO 80525-4417. Tel: 970-482-5868. Fax: 970-482-9735.
By Angelo DePalma
