Barr Urges Florida Lawmakers to Support Elimination of Negative Formulary

The report found that the "formulary committee's failure to affirmatively recommend... to delete generic drug products that have been subsequently designated... as therapeutically equivalent... is inconsistent with FDA bioequivalency standards."
"This report recommends sunsetting a 1976 law that is no longer necessary given the FDA's rigorous generic drug approval process," said Bruce L. Downey, Barr's chairman, president, and CEO. "We encourage the legislature to dissolve the Negative Formulary even sooner than proposed and bring Florida in line with 47 other states that accept the FDA's 'AB' rating as the gold standard for authorizing generic substitution." Among the 11 products on the Florida Negative Formulary is Warfarin Sodium, which has annual sales in the state of approximately $26 million.
Barr has for years been fighting against unreasonable laws preventing generic substitution. In fighting the good fight for its Warfarin Sodium product, Barr has raised consciousness regarding generic drugs from legislative halls to physician offices.

Barr's Warfarin Sodium, a generic version of Coumadin
Barr was a member of a coalition of business and consumer groups who worked with the Florida legislature during the 1999 session to draw attention to the millions of dollars being lost to taxpayers and consumers because of restrictions on the substitution of generic medicines. The report recognized that "pharmacy costs have become one of the fastest growing segments of overall health expenditures" and that generic drug substitution could help to control the growth of health care costs.
The report also noted that although it is possible to remove a product from the Negative formulary, the mechanism is susceptible to influence by drug manufacturers who have significant financial incentives to promote specific products. In February 1999, Barr temporarily withdrew its application to remove Warfarin Sodium from the Florida Negative Formulary, citing a relentless misinformation campaign by DuPont Pharmaceuticals Co., makers of the Coumadin brand anticoagulant. Barr's decision followed the rejection of the recommendation by the pharmacy consultant to the Formulary "that 'AB' rated Warfarin Sodium USP approved by the FDA be considered bioequivalent for interchange in patients requiring this medicinal product in the State of Florida."
Warfarin Sodium Tablets are used to treat complications associated with atrial fibrillation, a disease that affects 3 million Americans, particularly the elderly. To date, more than 6 million prescriptions have been dispensed with Barr's generic product.
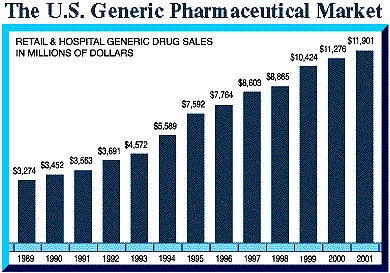
Generic pharmaceuticals represent a $7 billion industry, with generics representing one third (and growing) of the 450 million prescriptions filled annually in the U.S. Among the factors driving this growth are a greater emphasis on lower health care costs, the aging U.S. population, and the availability of hundreds of brand name pharmaceuticals that will come off-patent and face generic competition.
Coumadin is the 11th most prescribed medicine in the United States and has current annual sales of approximately $500 million. Barr received approval for its generic version of Coumadin on March 26, 1997, and introduced the product several months later.
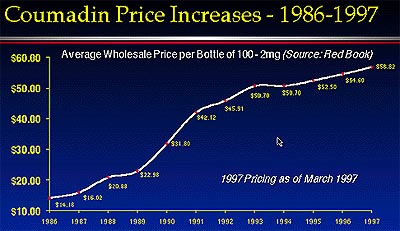
At the time, Barr Chairman Downey described the launch as "a milestone for Barr, and perhaps the most significant new product launch in the history of the industry." Strong words from a proud chief executive, perhaps, but not entirely without basis. According to Downey, the lack of competition for Coumadin resulted in three decades of monopoly pricing (see price graph). "Warfarin was discovered in the 1940s, and since its patent expiry in 1962, consumers have been held captive to ever increasing monopoly pricing. Today, consumers [of the brand-name Coumadin] are paying 300% more for the same bottle of medicine as ten years ago. Our Warfarin product will provide Coumadin patients—80% of whom are over age 60—with the same product at a substantial annual savings."
For more information: Bruce Downey, Chairman, Barr Laboratories, Inc., 2 Quaker Rd., Pomona, NY 10970. Tel: 914-362-1100.
By Angelo DePalma
