Best Practices To Ensure Quality Of Raw Materials Used To Manufacture Therapeutic Proteins
By Maura Kibbey, Ph.D., U.S. Pharmacopeia (USP)
Across biologics, raw material quality is a key driver of product quality and consistency. Assuring the quality of raw materials is, therefore, an important component of an overall control strategy. A manufacturer’s control strategy must ensure the quality of raw materials for producing therapeutic proteins even when supply chains are disrupted, such as during the COVID-19 pandemic.1,2
Despite and because of these challenges, manufacturers continue to leverage new and creative sourcing strategies, seeking new suppliers of ingredients such as resin supplies, reagents, and single-use systems. Securing high-quality raw materials is not the only challenge, however. Safeguarding the quality of acquired raw materials throughout storage is another essential element of a manufacturer’s quality management system. Recent reports demonstrate how improper handling can adversely impact the quality of biologics.3
USP’s Commitment To Raw Material Quality
USP4 has a long and sustained history of safeguarding the quality of raw materials used in biologics. The United States Pharmacopeia—National Formulary (USP–NF) contains several general chapters and monographs that describe quality attributes, tests, and acceptance criteria for raw materials and excipients. For example, USP <89> Enzymes Used as Ancillary Materials in Pharmaceutical Manufacturing includes quality attributes and associated tests and acceptance criteria for recombinant trypsin and other enzymes used in bioprocesses. Similarly, monographs in USP–NF, such as Polysorbate (PS) 80 and Polyethylene Glycol, contain tests and acceptance criteria that set the standard of quality.
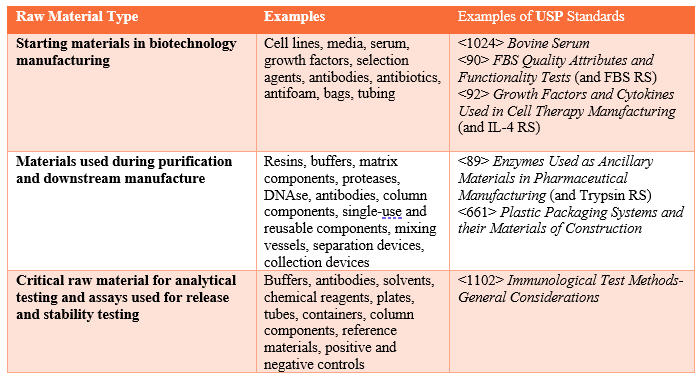
Manufacturers can use these compendial standards to build additional criteria to ensure materials are suitable for their purpose(s). Table 1 provides examples of raw material types and associated USP standards.
Table 1. Raw Material Types, Examples, and Associated USP Standards
In April 2021, USP continued its commitment to raw material quality by hosting a virtual workshop, Raw Materials for Manufacturing of Biologics: Best Practices and Quality Standards. The three-day workshop convened experts from diverse stakeholder groups, including manufacturers, suppliers, and regulators. In addition to regulatory perspectives, attendees from around the world gained valuable insights from industry experts who shared strategies and case studies reflecting best practices for ensuring quality, from raw material sourcing through product expiry. This workshop followed a March 2021 USP Center of Excellence training offered to regulators in the Asia-Pacific Economic Cooperation (APEC) region on the same topic but focused specifically on raw materials for advanced therapies. While both the APEC training and the April workshop focused on best practices for raw materials used in cell and gene therapy manufacturing, the principles around sourcing and qualification are similar to those for materials used in manufacturing recombinant proteins, which is the subject of this article.
Regulatory Perspectives: Qualification, Control, And Results from Inspections
Raw materials need to be qualified before manufacturing can begin. Therefore, manufacturers must provide regulators with information on their raw materials before they can start production. The information must be sufficient to assess the risks associated with raw materials and the resulting products. Therefore, raw material qualification and controls are important components of drug development.
These issues were discussed by representatives from the U.S. FDA during the April workshop. Ashutosh Rao, from the FDA’s Center for Drug Evaluation and Research, reviewed regulatory considerations for the qualification and control of raw materials used in biotechnology products. Rao shared that as defined in ICH Q7,5 raw material is “a general term used to denote starting materials, reagents, and solvents intended for use in the production of intermediates or [active pharmaceutical ingredients].” This broad category applies to diverse synthetic, organic, and mined substances, some of which are used for various applications across many different industries.
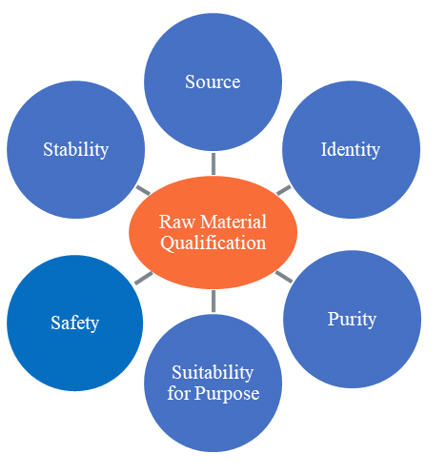
The identification and selection of raw materials should be based on their suitability for use and established through qualification and testing (Figure 1). Risk assessments conducted in early development can help manufacturers identify the criticality of materials and which of their attributes may impact critical quality attributes of the resulting therapeutic protein.
Figure 1. Considerations for Raw Material Qualification
Rao added that manufacturers of therapeutic proteins for the U.S. market must comply with the cGMP statutes in the Food, Drug, and Cosmetic Act and the cGMP regulations codified in the Code of Federal Regulations. Compliance with these laws is enforced through inspections carried out by the FDA. Inspections also serve a secondary purpose by providing the industry with valuable information about significant, frequent, and diverse manufacturing challenges. This information can be used to modify strategies to better meet cGMP requirements.
Ekaterina Allen from the FDA’s Center for Biologics Evaluation and Research shared recommendations on best practices from a compliance officer’s point of view. Allen presented FDA Form 483 observations illustrating a wide range of cGMP compliance issues related to biologics manufacturing and raw materials. Problems included inadequate inventory control, insufficient segregation, inadequate procedures to prevent incorrect raw material use, and the absence of established specifications or qualification of critical materials for their intended use. Verifying raw material identity, validating supplier analyses, and periodically conducting conformity testing remain important tools that help ensure raw material quality.
Standards, Methods, And Formulation Approaches That Support The Quality Of Polysorbates
Polysorbates are important excipients used in therapeutic protein formulations due to their ability to stabilize proteins by preventing aggregation and surface adsorption.6 However, PS degradation due to hydrolysis or oxidation can dramatically reduce its effectiveness in supporting therapeutic protein quality and safety. Over the last decade, USP has introduced significant updates to its PS20, PS40, and PS60 monographs to align them with improved knowledge and understanding of PS composition and complexity.
During the workshop, Richard Cawthorne and Dominic Moore, from USP’s Excipients Expert Committees (ECs) and the USP Joint Subcommittee on Polysorbates, reviewed the USP standards that help ensure the quality of PS excipients. USP’s Complex Excipients EC is currently developing a monograph for PS65. Additionally, USP’s ECs are developing and validating methods to directly analyze PS (PS20, PS40, PS60, and PS80) composition and identify their major components using intact PS. These efforts were recently published in a Stimuli7 article in the Pharmacopeial Forum to gather stakeholder feedback and to inform future PS methodologies and standards. Stakeholders are encouraged to submit feedback to USP.
While the complexity of PS composition poses challenges to their analysis, an extensive analytical toolbox for PS control for therapeutic proteins exists. Michael Jahn, head of forensic chemistry at Lonza, reviewed routine methods for monitoring PS content, quantity, and degradation in addition to special characterization methods that can support root cause investigations of PS degradation. Recent reporting demonstrated that 4-Hydroxynonenal (HNE) is an oxidative degradant of PS80, which raises safety and quality concerns due to its genotoxic potential and the possibility for α,β-unsaturated HNE to result in covalent protein modifications.8 However, the antioxidant butylhydroxytoluene has also been found to suppress HNE formation and therefore represents an important additive that manufacturers can use to help increase the stability of PS used in their formulations.8
Advanced Controls: Online Analytical Tools, Informatics, And Attribute-focused Approaches
Online analytical methods can help improve workflow efficiency and build a greater understanding of complex raw materials such as cell culture media components. Guarav Chauhan from Merck shared several strategies for implementing process analytical technologies (PAT). For example, manufacturers can visualize dissolution and improve mixing efficiency by using focused-beam reflectance measurements. Excitation emission matrix measurements can also be used to develop methods to quantify trace components of critical raw materials at the parts per billion level. Notably, manufacturers will develop a greater understanding of the impact of individual media components as the results from these techniques are added to process data repositories.
Online elemental monitoring is quickly becoming an important cell culture media control strategy, as trace metals in media can negatively impact the quality of therapeutic proteins. Advanced raw material characterization improves understanding of important quality attributes and the interactions between materials. With raw materials such as cell culture media formulations becoming increasingly complex, advanced tools are needed to help connect analytical data from suppliers, materials science, and in-house QC laboratories to manufacturing outcomes. Rapid identification, characterization, and online informatics tools can help make these connections. Thomas Matthews from Biogen presented a series of case studies demonstrating how to use data informatics and statistical systems to develop and implement PAT systems. In the future, these dynamic, enterprise-wide online tools could be leveraged to develop batch-specific predictions for diverse, increasingly complex raw materials.
Implementing a systematic and robust approach for identifying raw materials and controlling variability is essential to control process consistency and ensure product quality. Jacqueline Milne from Amgen reviewed the application of a quality target product profile approach for enhanced material attribute understanding and control. With this framework, a material target profile can be used to conduct a material attribute assessment, define a target attribute profile, and conduct a control assessment. This approach facilitates the development of science-based raw material specifications and phase-appropriate decisions across the life cycle of the materials. Digital information systems that monitor and control variability can enable manufacturers to routinely track trends and automatically relate raw material variation to process changes and product attributes.
Minimizing Risk And Supply Chain Disruptions: Strategic Planning, Technical Assessments, And Planning For Controls
Supply chain disruptions can impact not only biomanufacturing but also the quality of raw materials themselves. Strategies such as secondary sourcing help mitigate these risks. Min Zhang from Thermo Fisher Scientific outlined some raw material management strategies, including:
- Identify primary and secondary platform raw materials and specifications.
- Develop raw material management in early tech transfer.
- Evaluate alternative solutions.
In addition to secondary (or greater) sourcing, Zhang reviewed additional risk mitigation strategies, including securing manufacturing slot times and multiyear capacity and retaining additional stock.
As previously discussed, the control of trace elemental impurities remains a challenging yet critical aspect of raw material quality control. John Cunningham from Janssen reviewed two case studies that described strategies to control trace metals and a metal chelating agent. A critical, albeit underappreciated, endeavor is the development of a sample library that supports the testing of an array of samples before, during, and after an excursion. Cunningham also identified trust and transparency between suppliers and manufacturers as a foundational element for initiating and achieving a successful long-term control strategy.
Recognizing the importance of trace metals to the success of biomanufacturing, USP, in collaboration with BioPhorum, hosted a roundtable in 2019 on this topic in order to understand the challenges of evaluating these raw materials as well as their presence in more complex materials like cell culture media. Based on their findings, a new USP Expert Panel for Trace Metals in Cell Culture Media was formed in 2020 and is currently drafting an informational general chapter that will describe best practices, including risk-based approaches, sample considerations, commonly used methods based on inductively-coupled plasma techniques, and common isobaric and polyatomic interferences. The new chapter proposal should be published in the Pharmacopeial Forum in 2022.
The goal of qualification programs for raw materials is to enhance the pharmaceutical quality systems around manufacturing. USP is proud to be part of the quality and regulatory fabric that ensures the quality of medicines. We call on all stakeholders to reach out to us with suggestions for new topics for discussion, workshops, and standardization efforts via USPBiologics@usp.org. For more information about USP’s work in biologics, including standards that support raw material quality, visit USP.org/biologics. To view the talks released by the speakers and now available to the public, visit the USP Education portal.
About The Author:
 Maura Kibbey, Ph.D., is senior scientific fellow for education and training in USP’s Global Biologics department. She leads development of courses, workshops, and forums to engage USP’s biologics stakeholders. This role builds on her previous responsibilities directing USP scientists developing compendial standards. Before joining USP, Kibbey worked for several biotechnology and diagnostic companies in the Washington, D.C., area in scientific, management, marketing, and business development roles, as well as performing cancer research at the National Institutes of Health. She has published over 40 peer-reviewed articles and has been an invited speaker or workshop organizer for numerous scientific conferences. You can reach her at mck@usp.org.
Maura Kibbey, Ph.D., is senior scientific fellow for education and training in USP’s Global Biologics department. She leads development of courses, workshops, and forums to engage USP’s biologics stakeholders. This role builds on her previous responsibilities directing USP scientists developing compendial standards. Before joining USP, Kibbey worked for several biotechnology and diagnostic companies in the Washington, D.C., area in scientific, management, marketing, and business development roles, as well as performing cancer research at the National Institutes of Health. She has published over 40 peer-reviewed articles and has been an invited speaker or workshop organizer for numerous scientific conferences. You can reach her at mck@usp.org.
- https://www.pharmtech.com/view/ensuring-a-safe-and-robust-supply-of-pharma-materials
- https://cen.acs.org/business/outsourcing/COVID-19-reshaping-pharmaceutical-supply/98/i16
- https://www.fiercepharma.com/manufacturing/emergent-blasted-by-fda-for-quality-control-and-cleanliness-issues-at-troubled
- The US Pharmacopeia (USP) is an independent, nonprofit, scientific organization that collaborates with the world's top experts in health and science to develop quality standards for medicines, dietary supplements, and food ingredients. Through our standards, advocacy, and education, USP helps increase the availability of quality medicines, supplements, and food for billions of people worldwide.
- https://www.fda.gov/files/drugs/published/Q7-Good-Manufacturing-Practice-Guidance-for-Active-Pharmaceutical-Ingredients-Guidance-for-Industry.pdf
- Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci. 2008 Aug;97(8):2924-35. doi: 10.1002/jps.21190. PMID: 17973307.
- Understanding the Composition and Quality of Polysorbates to Strengthen USP–NF Compendial Standards. Pharmacopeial Forum. 47(1)[Jan.—Mar. 2021].
- Schröter A, Koulov AV, Huwyler J, Mahler HC, Jahn M. 4-Hydroxynonenal is An Oxidative Degradation Product of Polysorbate 80 [published online ahead of print, 2021 Feb 2]. J Pharm Sci. 2021;S0022-3549(21)00069-1. doi:10.1016/j.xphs.2021.01.027
