Can Diamond Make Drug Authentication "Flawless?"

By Dr. Andrew S. Janoff, Founder and CEO, Taaneh, Inc.
Pharmaceutical industry insiders estimate that more than $350 billion worth of counterfeit drugs are now sold around the world each year. In addition to diverting revenue from manufacturers and distributors, falsified medicines are also putting patients at risk. They are often not manufactured to even minimum quality standards, which can reduce their efficacy. In addition, in many cases they contain dangerous impurities that pose significant safety hazards.
Regulatory agencies around the world are taking action to try to address the problem. Several have recently introduced significantly higher standards for “track and trace” procedures to confirm authenticity. In 2011 the U.S. FDA published a new Guidance for Industry entitled “Incorporation of Physical Chemical Identifiers (PCIDs) into Solid Oral Dosage Form Drug Products for Anti-Counterfeiting.” In 2013 Congress then passed the Drug Quality and Security Act, which outlines the required steps manufacturers must take in order to establish acceptable systems to identify and trace prescription drugs through all phases of the distribution chain. Among its many requirements, the law details more stringent performance standards for authentication using options including traceable lot numbers, expiration dates, bar codes, and other unique identifiers. Even at this stage it is clear that manufacturers will soon need to identify and adopt advanced authentication technologies able to meet higher performance criteria in several areas, including:
- verifiable track and trace capability;
- advanced encryption that is difficult for others to replicate or decipher; and,
- real time mobile verification at any point in a supply chain using readily accessible technology.
Current Technologies In Drug Authentication
In recent years, manufacturers have focused on packaging options including serialization with the use of bar codes or other unique indicia to authenticate product. The reality is that options based on packaging alone are insufficient. They become useless in cases where the product is separated from its packaging.
To mitigate counterfeiting potential, authentication of both oral and infused drugs will require the use of traceable and readily-verifiable elements added to both formulations and packaging. Options might include the use of imprints, colorants, electronic microchips, and inert materials. But even among those additives, which do not require significant additional investments in technology or that will not disrupt manufacturing protocols, many present other limitations:
- Many identifiers do not provide a robust verifiable link between the packaging and the drug. They cannot unequivocally confirm that product and packaging are the same unit at every stage in the supply chain.
- Many identifiers are readily visible or easy to copy, increasing the risk that counterfeiters will be able to work around them.
- Materials added to formulations, even those that are inert, can present problems associated with content uniformity, affect manufacturing (for both oral and infused drugs), or limit printing capabilities.
- Bar codes or other identifiers added to the surface of oral therapies can require that drugs be reformulated with special coatings, which can increase costs and production time. In addition, bar codes are not an option for infused therapies.
For drug manufacturers, the ability to meet or exceed the new standards in authentication with a material/technology combination that is cost-effective, widely available, seamlessly incorporated into current manufacturing protocols, and easy to use would represent a major advancement.
Manufacturing With Diamond
Carbon is included on the FDA’s list of inactive ingredients and, as such, is considered acceptable as an additive to oral dosage forms. Diamond is an allotrope of carbon. Variations in the crystal lattice structure of diamond endow it with the ability to emit an almost-unlimited range of distinct spectral fingerprints that are impossible to replicate (Figure 1). In drugs, diamond particles can present these spectral identifiers even when present in only trace amounts. Based on these properties, the pharmaceutical industry has begun to investigate the option of adding diamond powder at low concentrations to drug formulations and to packaging and ink.
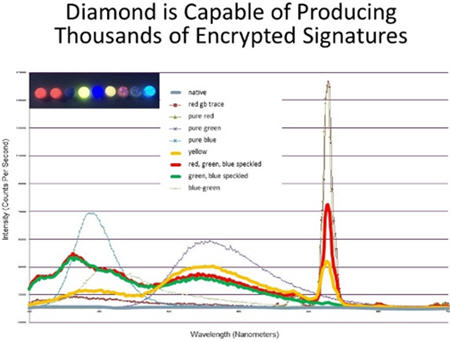
Figure 1
With the addition of diamond to drugs and packaging, manufacturers can use handheld detection devices to identify and confirm the spectral signatures unique to a drug or its packaging at any point in the distribution chain from manufacturing to consumer. Importantly, this makes it possible to confirm authenticity even when the drug is separated from its packaging. The unique spectral fingerprints of the authentic drug product and its packaging provide specific identifiers that are almost impossible to replicate. Handheld detectors programmed through a secure network can determine the spectral signature of product or packages and compare it to spectra in the database, confirming authenticity instantly (Figure 2).
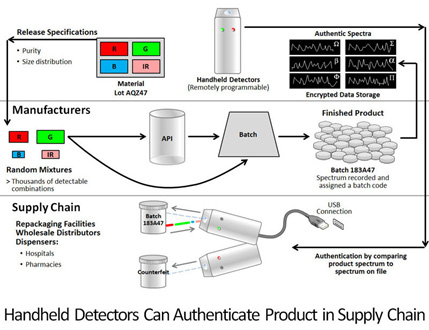
Figure 2
Diamond product authentication has also demonstrated another important advantage related to packaging. With this technology, packaging can be printed with ink containing the same random mixture of diamond that is added to the drug product. In addition to making it possible to authenticate drug and packaging independently, spectral signatures can also be observed directly through opaque packaging. Thus it creates options to confirm authenticity at the box, bundle, or unit level. [Figure 3].
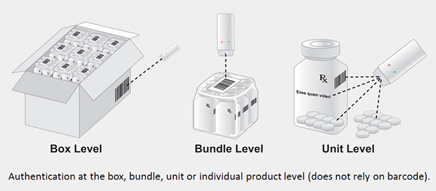
Figure 3
In manufacturing, diamond powder can be formulated to be consistent with established content uniformity criteria of ink, active pharmaceutical ingredients, and finished products. This technology can be employed without disrupting established printing and manufacturing equipment and protocols.
The first industrial use of diamond to prevent drug product and package counterfeiting is anticipated in the near future. Findings related to drug authentication are also raising interest in other industries affected by counterfeiting, and the use of diamond could prove valuable for manufacturers of OTC drugs, vitamins, and many processed foods.
