Cellegy Acquires Australia's Quay Pharmaceuticals
Through this deal, niche market-conscious Cellegy will gain more than just a one-product subsidiary. Quay provides Cellegy with a growing Australian sales and operating base, plus experience for commercializing nitroglycerin-based rectal products (trademarked Anogesic) in the United States and around the world. Demand for prescription and over-the-counter hemorrhoid and fissure treatments in Japan, the United States, and Europe is $475 million, with surgery for those conditions, according to Cellegy, worth several times that figure. The key dynamics for this niche market are that non-surgical treatments don't work, many patients do not seek treatment because of embarassment or fear, and those who do (as well as their insurers) strongly prefer to delay or avoid surgery. Rectogesic (nitroglycerin) is a smooth muscle agent that relaxes the anal sphincter, allowing blood flow to resume to affected areas. Because it works indirectly on blood vessels involved in bleeding, the medication gives affected areas the chance to heal on their own.
"We believe in Anogesic's sales potential and Cellegy management's ability to maximize returns in the international marketplace," commented David Lubowski, founder of Quay and a prominent colorectal surgeon. "Rectogesic has been enthusiastically embraced by Australian surgeons since its launch and its use caused a dramatic fall in the number of sphincterotomies (surgical interventions) performed in my own practice." Lubowski did not mince words: "The introduction of Rectogesic for treating anal fissures is analogous to the virtual elimination of peptic ulcer surgery following the introduction of Tagamet in the 1970s."
Lubowski also believes Rectogesic and Anogesic will be approved for other indications, including hemorrhoids and pain after hemorrhoid surgery. The Australian Board of Health approved Rectogesic following the usual three-year review of a regulatory submission made by Quay. Products approved by the Australian Board are considered by many foreign regulatory authorities to be sufficiently safe and effective to merit marketing approval in their own countries.
Cellegy is now busy preparing marketing applications in several countries, according to CEO K. Michael Forrest. "We are confident that several approvals will be received well in advance of the timetable which would have been required based on approval by the FDA, thereby allowing for an earlier revenue stream. A total of 2.5 million people suffering from anal fissures or hemorrhoids in target markets could benefit from the early availability of Anogesic." Forrest was referring to South Africa and several countries in Latin American and East Asia (excluding Japan) that he believes will accept the Australian studies and package for Rectogesic without further clinical trials.
In Australia, Rectogesic is technically an over-the-counter medication, but it can only be sold by a pharmacist. In the United States, the product would be available by prescription only.
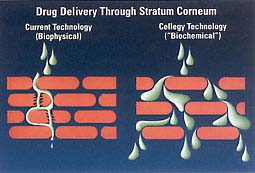
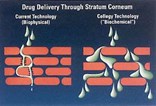
Cellegy's Permeate technology, used in the company's skin care products, uses permeation enhancers to permit controlled passage of large molecule drugs through the skin. Permeate enables transdermal drug delivery without the need for patches or electrical stimulation (iontophoresis) or ultrasound to drive drugs through the skin barrier.
Cellegy Pharmaceuticals is a specialty biopharmaceutical specializing in skin care and prescription drugs. Phase III testing of Anogesic for anal fissures is ongoing and is expected to permit an NDA submission in 2001. Cellegy is also conducting two Phase II clinical trials using Anogesic to treat hemorrhoids, a condition which afflicts over 25 million people worldwide. Cellegy's other prescription products include two transdermal testosterone drugs: Tostrex (testosterone gel) for male hypogonadism; Tostrelle (testosterone gel) for decreased libido in postmenopausal women. Tostrelle is currently undergoing a Phase I/II dose ranging study in the United States. Cellegy has also developed a line of high performance cosmeceutical products, which have been shown in
clinical tests to produce significant improvements in the appearance of photodamaged and wrinkled skin.
For more information: Richard Juelis, VP of Finance, Cellegy Pharmaceuticals, Inc., 349 Oyster Point Boulevard, Suite 200, South San Francisco, CA 94080. Tel: 650-616-2200.
By Angelo DePalma
