Chargepoint Technology And Famat Sampling To Develop Containment Solutions For HPAPI Powder Transfer
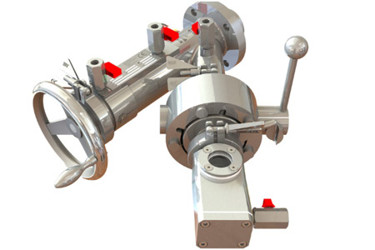
Powder transfer specialist, ChargePoint Technology, has joined forces with sampling device specialist, Famat Sampling, to address one of the industry’s most challenging containment problems.
The pharmaceutical suppliers have created an integrated valve solution capable of allowing operators to take a product sample during the course of the production process for quality analysis while maintaining optimum containment and sterile integrity.
The combination of ChargePoint Technology’s split butterfly valve and Famat Sampling’s 125-TC/OEL valve creates a secure and sterile environment for high potency and sterile powder samples to be taken. The interconnected system forms an airtight seal that prevents a dead zone and allows samples of highly potent, toxic, or sterile powders to be transferred into containers safely and securely, maintaining OEB4 and OEB5 level compliance.
In addition, the integrated network of valves allows for rigorous testing capabilities and ensure that product quality is maintained during the process and it is FDA compliant. The combination of products help minimize risk to the operator, who could be exposed to potentially dangerous powders during the sampling process.
The powder product must not be altered during sampling, as oxygen or other particles could come into contact during the extraction process and damage the product. The combination of technologies from ChargePoint Technology and Famat acts as an isolator and offers users a safe method of transfer so the quality of the product can be protected.
Christian Dunne, head of sterile solutions at ChargePoint Technology, said, “The HPAPI market is predicted to grow by 8.7% to reach a value of $34.97 billion by 2028, highlighting why it’s vital for drug manufacturers to invest in appropriate containment technologies, as while ensuring they are able to maintain optimum product quality.”
Dunne added, “During processing, manufacturers must prioritize sampling so that the quality of drug products can be assessed at various stages of the production process. Famat Sampling shares our high standards for quality. Joining forces as two recognized industry leaders in our respective fields was a no-brainer for our team. Our shared depth and breadth of knowledge and experience have allowed us to create a solution that helps solve a prevalent issue for manufacturers and adds additional protection for users.”
Source: Chargepoint Technology
