Cold Box Express, LLC Completes And Passes Comprehensive 3rd Party IQ/OQ And PQ Validation Protocol
One of the main purposes for these cold chain performance qualification tests is to ensure the shipper performs as expected under simulated real-world conditions. The validation included distributing and affixing 22 Sensitech TempTale (TT4) internal probes and temperature loggers inside the payload compartment to determine if the box is capable of maintaining a consistent set temperature point during both typical transport scenarios. Worst-case scenarios include extreme ambient conditions, power outages, an improperly closed or sealed door, or an empty payload compartment.
These types of validation tests are particularly important for those in the pharmaceutical industry. With such high risk involved in shipping temperature sensitive cargo such as active pharmaceutical ingredients, vaccines, or biological materials, the insurance companies of many pharmaceutical businesses will not permit shipments without validation through strict performance qualification testing. This pharmaceutical cold chain validation thus ensures the shipping and distribution process will offer no negative impact to the safety, efficacy or quality of the biological or pharmaceutical products. Negative impacts that can compromise the temperature controlled pharmaceutical shipments include any irregularities in cooling, warming, and/or freezing due to external temperature changes, shipment mishandling, or improper packaging.
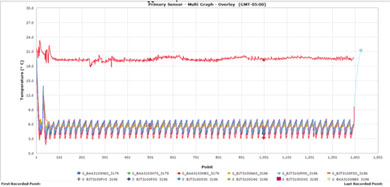 The 3rd party validation test had a set temperature at 41°F or 5°C ±3°C, while temperature loggers were programmed to record every 5 minutes for 60 hours. These temperature TT4 loggers were strategically placed in the interior payload compartment. The Cold Box shipper completed and passed all of the validation testing including IQ/OQ and PQ protocols.
The 3rd party validation test had a set temperature at 41°F or 5°C ±3°C, while temperature loggers were programmed to record every 5 minutes for 60 hours. These temperature TT4 loggers were strategically placed in the interior payload compartment. The Cold Box shipper completed and passed all of the validation testing including IQ/OQ and PQ protocols.
For more information on using Cold Box for your pharmaceutical cold chain shipping, please visit: http://thecoldbox.com/pharmaceutical-shipping/
Source: Cold Box Express, LLC
