100% Container Closure Inspection Of Freeze Dried Drug Product In Quarantine
Source: Lighthouse Instruments
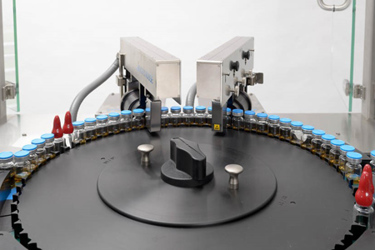
Introduction
Lyophilization is a complex process that presents many manufacturing challenges, one of which is maintaining and monitoring container closure integrity of the finished package. Container closure integrity plays an important role in maintaining the sterility and stability of lyophilized products. Concerns over patient safety, customer complaints, and the cost of investigations and product recalls have recently resulted in revised regulatory guidance (e.g. the revised Annex 1 of the European Guidelines for the Manufacture of Sterile Products).
access the Case Study!
Log In
Get unlimited access to:
Trend and Thought Leadership Articles

Case Studies & White Papers

Extensive Product Database

Members-Only Premium Content

Welcome Back! Please Log In to Continue.
X
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
Subscribe to Pharmaceutical Online
X
Subscribe to Pharmaceutical Online
