Design Space Development — How (And When) To Get Started
By Sandra Wassink, Principal Process Engineer, Pharmatech Associates
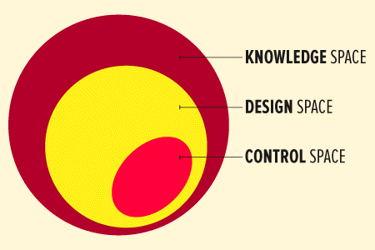
Design space is a scientific concept used in the pharmaceutical/biopharmaceutical industry to support and assure product quality. The culmination of the information and knowledge gained during product development provides the foundation for the design space. The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) defines design space as the multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality.1
In early-stage product development, the challenge of finding the optimal design space is determining what is important to achieve consistent product quality. A stepwise approach should be taken in the formulation and development process to capture characteristics or attributes that could impact product quality. Key areas of focus are materials — active pharmaceutical ingredients (APIs) and inactive components (excipients) — manufacturing process, and the desired quality target product profile (QTPP) tolerances and process variation variables.
A company sponsoring the development of a new product should draft the product objectives in a target product profile (TPP). A TPP helps focus the drug development team on goals in terms of drug labeling, and assists in addressing issues early in the drug development process. Drug labeling concepts to consider during development are dosage and administration, and dosage form and strengths. The development program should be focused on determining the quality attributes to meet the quality TPP.
When defining design space, your approach should include target product critical quality attributes (CQAs), prior scientific knowledge, and risk assessment. Critical quality attributes are the properties or characteristics that should be within an appropriate limit, range, or distribution to ensure the desired product quality. Implementing quality risk management2 and quality by design (QbD)1 methodologies as early as possible will help define what is critical and will facilitate defining the design space. Factors that impact the development of design space are financial constraints, staff resources, and timing to market. Once the decision is made to establish design space, using knowledge management in combination with risk management and QbD techniques will facilitate the development of the design space.
Establish a Knowledge Management System
One of the first steps to establishing design space is to determine a process for capturing information and scientific knowledge. In this context, knowledge management is the systematic approach to acquiring, analyzing, storing, and disseminating information related to products, manufacturing processes, and components.3 Once a knowledge management process is created, existing data, prior knowledge, and new information can be added to the database. One of the simplest tools for knowledge management is the spreadsheet, which can be used to collect information for the database. As information is generated, it should be documented in the database and evaluated.
Risk assessment should be conducted as part of the evaluation to determine if the new information is critical and has an impact on product quality. Using a QbD approach during the product development will help identify and determine the relationship between the materials and manufacturing process that impacts the product quality attributes. Using this approach establishes what we call “compliance through science” and is the foundation for ensuring a safe, quality product.
Evaluate Active and Inactive Components
The first key area to assess concerns the materials/components used in the drug product. Suppliers should provide certificates of analysis that include the acceptance criteria or specifications, test methods, and test results for the materials. This data should be added to the knowledge management database and evaluated to determine if any of the material characteristics has an impact on product performance. Material attributes of the API/drug substance and inactive components can influence the performance of the drug product and manufacturing process. For API, the physicochemical and biological properties should be identified. For the inactive components evaluated, their concentration and characteristics can also influence drug product performance or manufacturability. Information on excipient performance can be used to justify the choice and quality attributes of the material and to support the drug product specification. Implementing formal experimental design will make it easier to determine the critical material attributes that influence product quality.
Assess Manufacturing Process Parameters
The second key area to evaluate is the manufacturing process. An initial risk assessment of the manufacturing process steps should be performed prior to the baseline characterization work, in order to determine potential critical process steps. Critical process parameters (CPP) are those whose variability has an impact on a critical quality attribute — therefore, these should be monitored or controlled to ensure the process produces the desired quality. This assessment, in combination with the prior scientific knowledge, can be used as the primary means of determining the criticality of process parameters under the following conditions:
- When a platform process that possesses similar properties and process is applied to another commercial product (e.g., new strength or new dosage form)
- When there is a significant body of published data on the process
- When experimental studies and commercial data are available, such as when the process validation lifecycle is applied to a legacy product to substantiate the initial assessment4
Manufacturing process development and determination of critical process parameters will be influenced by the components. By overlaying the component CQAs with the process parameters, you can then determine the critical process parameters that impact product quality. Experimental design should be implemented to identify the relationship between materials and process parameters that impact product quality. The manufacturing process development program should identify any critical process parameters to be monitored or controlled to ensure desired product quality.
Review Attributes for Criticality
After preliminary material and process attributes are identified, they can be reviewed to determine criticality and the impact on the QTPP. Tolerances around the critical attributes and critical process parameters can help define the product specification limits.
In summary, the steps for establishing design space include: (1) develop a knowledge management and risk assessment process, and (2) evaluate and determine critical API and inactive material attributes and manufacturing process parameters by using QbD techniques.
Using a stepwise and methodical approach during early-stage product development will give you a good start on establishing the design space for the product. The development and definition of the final design space will continue through later product development stages. Ultimately, the design space should be defined before starting the first stage of process validation — process design.5
Conclusion
It is important to begin to define design space as soon as possible in early-stage product development. By using a risk-based approach, knowledge management, and QbD methodologies, the critical material, process parameters, and product quality attributes will be identified. Implementing these approaches establishes compliance through science and helps ensure product safety and quality. In the later stages of development, continued use of these approaches can be used to support process design and commercial product development. Ultimately, the goal is to define design space and submit the information to the relevant regulatory agencies to support potential changes that may occur during the commercial product lifecycle.
References:
- International Conference on Harmonization (ICH) and FDA Guidance for Industry, Q8 (R2) Pharmaceutical Development, Nov. 2009.
- ICH and FDA Guidance for Industry, Q9 Quality Risk Management, June 2006.
- ICH and FDA Guidance for Industry, Q10 Pharmaceutical Quality System, April 2009.
- Mitchell, Mark, Determining Criticality-Process Parameters and Quality Attributes Part I: Criticality as a Continuum, BioPharm International, Vol. 26, Issue 12, Dec. 2013.
- FDA Guidance for Industry, Process Validation: General Principles and Practices, Jan. 2011.
About The Author:
 Sandra Wassink, Pharmatech Associates’ principal process engineer, has worked in the regulated life sciences industry for over 30 years. With her technical expertise in QbD and knowledge of global regulatory requirements, she serves as a high-level resource in product and process development and regulatory compliance on numerous client sites and projects for the consultancy. Ms. Wassink holds a B.S. degree in biology with a minor in chemistry from Florida State University.
Sandra Wassink, Pharmatech Associates’ principal process engineer, has worked in the regulated life sciences industry for over 30 years. With her technical expertise in QbD and knowledge of global regulatory requirements, she serves as a high-level resource in product and process development and regulatory compliance on numerous client sites and projects for the consultancy. Ms. Wassink holds a B.S. degree in biology with a minor in chemistry from Florida State University.
