Digital Connected Manufacturing Network (CMN) For Pharmaceutical Manufacturing

Connect your entire operations across internal and external sites
Product Lifecycle Data
Consolidate data for each stage of a drug’s lifecycle in one place.
- Expedite compliance by centralizing product lifecycle data for each stage
- Shorten the time between one stage to the next
- Streamline tech transfer and accelerate to commercial rollout
Enterprise Recipe Management
Optimize enterprise recipe management across environments and sites.
- Create and manage a core set of enterprise-wide templates
- Get access to customizable resources for every stage
- Easily assign your templates and resources to individual sites and teams
External Manufacturing Collaboration
Ditch email and calls for automated communications.
- Grant direct view-only access and batch review options as needed
- Provide customers with a self-service approach to their sponsored batches
- Make real-time, data-driven decisions
Tech Transfer
Quickly and easily move all your master resources and data.
- Securely store batch templates, procedures, materials and equipment classes
- Easily share process knowledge and data through transferable global recipes
- Shift a product ad-hoc from one team to another
Manage capacity and execution across multiple internal and external manufacturing sites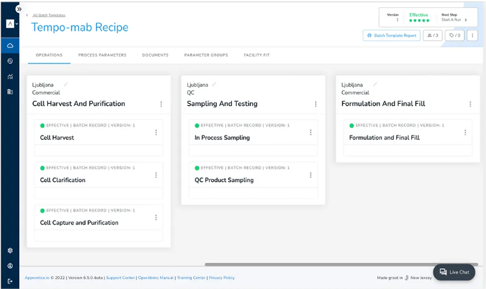
Speed up manufacturing with multi-team execution
Accelerate batch processing by allowing multiple teams to operate from a shared batch record and execute different steps in parallel. Associated teams can access recipes, tag comments, attach documents, and manage approvals, maintaining compliance requirements.
Harmonize processes and data between sites with a single shared system
Eliminate manual data transfer delays between environments with instantaneous digital transfer of all master data and templates. Safely share access to master data with role-based permissions.
Facilitate global collaboration between internal sites and external partners
Access operations at any team and any site you’re authorized for — internal or external — as an access-granted Tempo user. Get a holistic view of multi-product, multi-site operations with real-time updates on all the moving parts.
Accelerate product pipeline with centralized recipe management and optimization
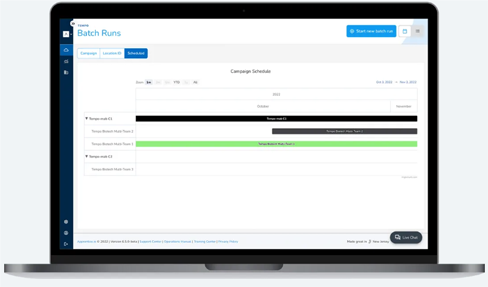 Compress your lifecycle stages, from preclinical to commercial
Compress your lifecycle stages, from preclinical to commercial
Shorten your product lifecycle stages through vertical integration of your systems. Reduce hand-offs between stages by granting universal access to contextualized data from different systems. Eliminate the need for individualized training on multiple systems or manual data transfer between point solutions, resulting in fewer delays and errors.
Reduce cycle time by acting on real-time data
Update and track recipe changes in real-time, providing a clear audit trail that automatically displays the impacts of each change. Respond instantaneously to production changes by planning and tracking batch timelines with scheduling.
Centralize global enterprise recipe management
Standardize product recipes such as batch and procedure templates for accuracy and uniformity throughout your operations. Conduct recipe transfers between teams and environments from one lifecycle stage to the next.
Expedite new product introduction by digitally conducting technical transfer
Quickly scale products with digital tech transfers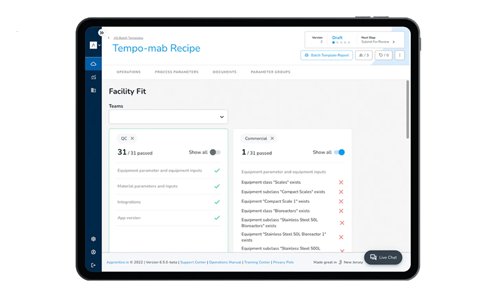
Expedite the slowest part of the therapeutic development pipeline with centralized management of product lifecycle data. Share a product’s manufacturing data, knowledge, processes, and technology between different stages and teams.
Enhance flexibility and scalability of products
Scale optimized recipes efficiently with templatized golden runs and batches. Use a single, distributable, source of truth for golden batch recipes with enterprise recipe templates that can be set up quickly at a new site.
Collaborate in real time with seamless data exchange
Streamline new product introduction by bringing all the fragmented parts, processes, and people into a single unified platform. Maintain regulatory standards while remaining agile with full audit trail, electronic signature records, version management, and role-based access from anywhere around the world.
Share real-time visibility with external partners through controlled digital access
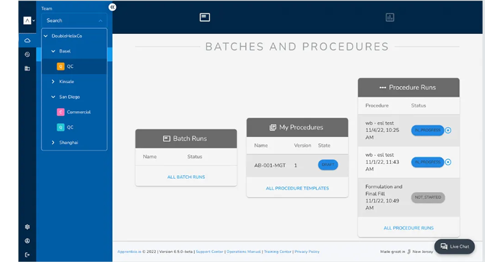 Collaborate transparently with batch run reviews
Collaborate transparently with batch run reviews
Provide external partners access to their production data and collaborate on reviews and approvals. Allow partners to assess quality throughout the manufacturing process with review access to ensure that their compliance requirements are met the first time around.
Ensure accurate contract manufacturing execution
Enable external partners to view batch and procedure data through an assigned External Sponsor Role. Share real-time updates about runs with your partners and streamline the process of coordinating the production and release of batches on a single integrated system.
Increase customer trust with end-to-end visibility
Resolve issues faster by identifying blockers and acting on them in real time. Provide a true partnership to your partners by coordinating execution and batch release with built-in review and customizable approval permissions.
