Eastman cGMP Intermediates Plant Will Build on Epoxybutene Technology
Contents
Two Production Lines
EPB
Strategy
Large plants are usually news, but Eastman Chemical Co. (Kingsport, TN) is building a small facility that should break some ground in the pharmaceutical intermediates business.
Eastman has begun construction of a new current good manufacturing practices (cGMP) facility at Batesville, AR. cGMP designates plants that meet the pharmaceutical industry's strict purity and materials tracking requirements.
The plant will manufacture pharmaceutical intermediates and bulk actives in quantities ranging from a few grams to metric tons Kvaerner Engineering (Bridgewater, NJ) will handle plant design, construction, and validation. Eastman expects the unit to reach full capacity at the start of the fourth quarter of 2000.
It is the first of two plants Eastman envisions for the site. The company hopes to use its new 3,4-epoxy-1-butene (epoxybutene) small molecule chemistry to provide cost-effective routes to new pharmaceutical intermediates.
Two Production Lines
"There's a need for US-based cGMP capacity," says Eastman commercial development representative John Maddox. "There is lots of demand from US pharmaceutical companies, but most capacity is in Europe. Our typical clients are major pharmaceutical companies that have decided their core competencies don't lie in manufacturing intermediates. They would rather outsource production to fine chemical companies."
The plant will house two production lines. The first will use a 22 L reaction system to produce small amounts of new chemical entities (NCEs) for clinical testing. It will start up during the second quarter of 2000.
The larger portion of the plant will consist of a mix of 100 gal and 300 gal reaction vessels. The unit's capacity will range from a few kilograms to metric ton amounts of cGMP pharmaceutical intermediates and bulk intermediates. It will also permit Eastman to conduct a variety of organic syntheses, including hydrogenation, halogenation, and low-temperature reactions.
Plant size matters, especially the ability to make both small and large amounts of product. "When pharmaceutical companies develop new products, they need kilograms for clinical trails and tons for final products," Maddox explains.
"To obtain large-scale business, we have to have facilities to supply products for clinical testing as well as. We are responding to their requests. Lots of chemical suppliers have capacity to supply small quantities, but can't expand to provide multiton amounts. Eastman will be able to ramp up with them and stay their supplier all through the development chain," Maddox concludes.
Eastman can scale up customers' process routes or design the routes itself. One key company strength, says Maddox, lies in Eastman's understanding of photographic intermediates. "They're both heterocyclic compounds with similar chemical transformations and high purity specifications," he explains.

The Texas Eastman plant at Longview, TX, has capacity for 3 million lb/yr of EPB oxirane. The company also operates production sites in Tennessee, Arkansas, and Wales (UK).
EPB
Eastman hopes to build many intermediates on its 3,4-epoxy-1-butene (epoxybutene, or EPB) family of small molecules. Eastman synthesizes the molecules using a proprietary process to continuously oxidize butadiene in air. The technology, which Eastman describes as the world's most selective vapor-phase air oxidation process, makes EPB production economical for the first time.
"From that single molecule, we derive a wealth of small molecule derivatives that offer cost-advantaged routes into several small-molecule intermediates, such as cyclopropyl intermediates, used in pharmaceuticals and agricultural chemicals," says Maddox.
Eastman views EPB as a multifunctional molecule, with every carbon atom chemically nonequivalent. It is able to modify the epoxide and vinyl groups individually, or simultaneously (which is possible because of conjugation of the olefin and epoxide groups). As a four- carbon molecule, epoxybutene readily leads to the production of chemicals traditionally obtained from Reppe chemistry (via acetylene and formaldehyde).
Isomerizing EPB oxirane yields 2,5-dihydrofuran (2,5-DHF), a intermediate used in furan chemistry. Thermally isomerizing 2-5-DHF yields cyclopropanecarboxaldehyde (CPCA), a starting aldehyde for cyclopropyls used in pharmaceutical and agrichemical applications.
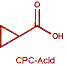
Cyclopropanecarboxylic acid (CPC-Acid)
Eastman has the ability to produce several key CPCA-derived cyclopropanes. Air oxidation of CPCA yields cyclopropanecarboxylic acid (CPC-acid). CPC-acid, in turn, is the starting point for methyl cyclopropanecarboxylate (MCPC), and possibly other alkyl esters as well. Reduction of CPCA yields cyclopropanemethanol (CPMO).
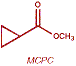
Methyl cyclopropanecarboxylate (MCPC)

Cyclopropanemethanol (CPMO)
Other EPB reactions promise additional chemical flexibility. Reactions on the epoxide ring leave EPB's vinyl group available for further elaboration or polymerization. Eastman notes several reactions: addition of water to form allylic acids; addition of alcohols to create allylic ethers; attachment of anhydrides to yield diesters; and addition of carbon dioxide to form vinyl ethylene carbonate (a potentially useful new monomer).
Additions to the EPB oxirane double bond create new products. Hydrogenation, for example, yields butylene oxide, an epoxide used in polyols and disinfectants. Halogenation produces molecules with uses analogous to epichlorohydrin and epibromohydrin.
Strategy
Eastman's thrust into life sciences chemistry, which includes pharmaceuticals and agrichemicals, is relatively recent. The company expanded its cGMP business with the acquisitions of Solvay Duphar in Wales. In August, it completed conversion of a plant in Hong Kong into a cGMP facility.
Eastman sees the life sciences platform as a major growth vehicle, and plans to expand its presence worldwide. Competitors capable of providing both small and large volumes of pharmaceutical intermediates include Catalytica and Lonza.
"This investment by Eastman enhances our cGMP capabilities in the United States and complements our cGMP manufacturing facilities in Europe and Asia Pacific," says Rob Hornsey, VP/GM of Eastman's Fine Chemicals business. "It shows our continued support of the pharmaceutical industry by providing state-of-the-art facilities to meet the industry's outsourcing needs for US cGMP manufacturing capacity."
"The small-scale plant is the first investment of our two-phase cGMP North American expansion effort," adds Roger Wright, president of Eastman's Arkansas operations. "This plant precedes the construction of a commercial-scale cGMP plant capable of manufacturing multiple metric tons of pharmaceutical intermediates and bulk actives."
For more information: John Maddox, Commercial Development Representative, Fine Chemicals, Eastman Chemical Co., PO Box 511, Kingsport, TN 37662. Phone: 423-229-4227. Fax: 423-229- 8133.
By Alan S. Brown
