Engineering Approaches To Respiratory Drug Delivery: Mannitol Case Study
By Cameron Kadleck, Respiratory Scientist, Product Development, Jimmy Beaty, Respiratory Scientist, Product Development, Matthew Ferguson, Ph.D., Senior Principal Investigator
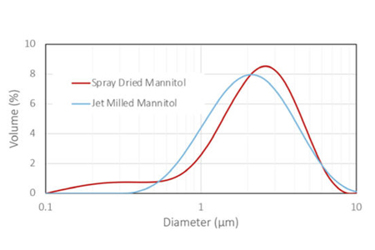
Spray drying and jet milling are commercially viable engineering processes for the development of respirable drug products. This article presents a case study that explores the material and performance properties of spray-dried and jet-milled mannitol for respiratory delivery of crystalline mannitol. The head-to-head comparison reveals opportunities and risks for designing a product based on each approach. It also explores the pros and cons of spray drying versus jet milling, describes how to use risk assessments to inform engineering technology selection, and illustrates how particle morphology affects aerodynamic performance.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
