Ensuring Repeatable, Viable Surface Sampling In Aseptic Settings
By Josh Erickson, senior director, microbiology pipeline and operations, United States Pharmacopeia

Viable environmental monitoring (EM) involves detecting and enumerating microorganisms in a cleanroom or controlled environment, providing insight into microbial control within the facility.1 For both cGMP facilities and sterile compounding pharmacies, EM is fundamental in ensuring that environmental conditions do not compromise the quality of the therapeutic products produced.
Monitoring viable particulates in the air and on surfaces helps identify risks, enabling timely interventions that support compliance with regulatory requirements like United States Pharmacopeia (USP) Chapter <797> Pharmaceutical Compounding — Sterile Preparations, good FDA cGMPs, and Annex 1: Manufacture of Sterile Medicinal Products of the EU GMP guidelines.2,3
What Is Surface Sampling?
Surface sampling is a key component of EM programs designed to monitor the microbial bioburden on surfaces in ISO classified or controlled environments. It involves collecting microbial samples from specific, risk-based locations, using contact plates or swabs. Surface sampling is essential for both pharmaceutical manufacturers and sterile compounding facilities, offering critical insights into environmental contamination levels and supporting proactive measures to maintain acceptable conditions.
Why Surface Sampling Matters
The data generated through viable surface sampling forms the foundation of an effective EM program. These insights are vital for:
- ensuring the safety and quality of sterile products,
- meeting regulatory requirements, and
- identifying and mitigating contamination risks to protect patient safety.
Without accurate and repeatable data, the surface sampling simply becomes another task that is completed to check a regulatory compliance box. Data must be viewed as an informational tool to aid in assessing the effectiveness of the facility’s contamination control strategy (CCS).
Surface Sampling Limitations
While surface sampling is invaluable, it is not perfect. USP General Chapter <1116> Microbiological Control and Monitoring of Aseptic Processing Environments highlights these limitations.4
- Surface sampling cannot prove or disprove the sterility of a product or preparation. The data collected can only help ensure that a system or workflow is in a consistent state of control.
- Surface sampling is semiquantitative. It is not possible to sample all surfaces or ensure recovery of every microorganism present, as the media and incubation parameter used are typically focused on the recovery of mesophilic aerobic microorganisms.
- No surface sampling plan can prove the absence of microbial contamination. No recovery of microorganisms only means that growth was not discovered and not that the cleanroom or controlled environment is free of contamination.
Recovery efficiency is highly dependent on the sample collection method, technique of the personnel collecting the sample, the sample collection device used, and environmental conditions. Understanding and mitigating these limitations through proper training and the use of validated methods is critical.
Applications In Aseptic Environments
Viable surface sampling is pivotal in:
- Aseptic pharmaceutical manufacturing: European Union (EU) GMP Annex 1 and other industry guidance documents emphasize the importance of surface sampling being part of the EM program and its role in validating facility design and processes both initially and ongoing. USP <1116> provides guidance on the use of viable surface monitoring to support contamination control strategies in controlled environments and further emphasizes the importance of personnel training for microbial monitoring activities.
- Outsourcing facilities: These unique organizations follow modified GMPs, while producing sterile preparations on a large scale. The purpose of surface sample collection and the EM program in an outsourcing facility is the same as in aseptic manufacturing environments.
- Traditional Sterile Compounding Pharmacies: USP Chapter <797> Pharmaceutical Compounding — Sterile Preparations requires viable sample collection to ensure an ongoing microbial state of control.
Key Considerations for Implementing A Robust EM Program
Sampling frequency and location
Regular monitoring in critical zones, as well as routine checks of less critical areas, helps to establish a comprehensive profile of the cleanroom environment. Annex 1, USP <797>, and other industry documents provide guidance on the areas to prioritize, such as ISO Class 5 environments and cleanroom areas, ensuring that sample sites are carefully chosen for their impact on product and preparation quality.
Standard operating procedures (SOPs)
SOPs should provide detailed instructions for viable EM sampling, incubation, and data recording. Consistency in methodology and adherence to SOPs are foundational to reliable EM data and regulatory compliance.
Continuous training programs
Initial and ongoing training for personnel conducting EM are essential to address technique, aseptic behavior, and the specific requirements cGMP manufacturing environments. Facilities should document training, assess competency regularly, and provide additional training as new EM methods or technologies are introduced.
Quality oversight and documentation
Oversight of EM activities, coupled with meticulous documentation, is essential for internal quality assurance and regulatory audits. Detailed records of EM results, deviations, and corrective actions are required to demonstrate compliance, monitor trends, and implement timely responses to environmental issues.
The Importance Of Consistent And Repeatable Surface Sampling Data
Enhancing product and preparation safety and quality
A well-executed EM program is essential to proactively address contamination before it impacts product quality. Consistent surface sampling helps establish baseline environmental conditions and provides insights into any variations in microbial presence. Identifying changes promptly is essential to prevent potential product and preparation contamination, ensuring patient safety and regulatory compliance.
Meeting regulatory standards
Many regulatory requirements mandate surface sampling in sterile compounding and cGMP facilities, outlining specific requirements for frequency, methods, and sample locations. Consistency and repeatability in EM practices are necessary, allowing facilities to demonstrate their commitment to microbial control and data integrity.
Facilitating root cause analysis
In cases of deviations or environmental excursions, repeatable surface sampling data can help facilities determine the source of contamination. By establishing a trend of historical data, teams can identify contamination patterns, assess potential corrective actions, and implement preventive measures. Without consistent EM practices, identifying contamination sources and implementing corrective actions becomes difficult, putting facility compliance and product/preparation quality at risk.
Why Personnel Collecting Samples Need To Be Trained And Qualified
Ensuring accurate and reliable data
Given the inherent limitations of surface sampling due to the nature of the test, facilities must prioritize consistency in their sampling practices to ensure accurate and reliable data. This consistency begins with having qualified personnel who can handle samples and media without introducing contamination. It is equally important to minimize recovery variability in collection techniques among those collecting samples. Achieving proper technique in surface sampling requires rigorous training and ongoing evaluation to uphold high standards and ensure uniformity.
Compliance with industry standards
Industry standards and guidance documents specify the need for qualified personnel. USP <797> states that “it is important that personnel are trained and competent in air and surface sampling procedures to ensure accurate and reproducible sampling.” Annex 1 emphasizes the importance of personnel qualification and microbial recovery efficiency. Clause 7.1 specifies that personnel must be “suitably qualified, trained and experienced” in any of the site’s operations. This would include viable surface sample collection.
Supporting effective data analysis
Qualified personnel are better equipped to collect samples consistently, minimizing variability due to handling and sampling techniques. Reliable sample collection is integral to obtaining representative data, which in turn supports meaningful trend analysis and the overall effectiveness of the EM program. Inaccurate or inconsistent sampling by untrained personnel can lead to misleading results, compromising the facility’s EM program and response to potential adverse trends.
Letting data be the guide
The importance of microbial surface sampling programs, along with having trained and competent personnel, cannot be overstated. Demonstrating that personnel are effectively recovering microorganisms from surface samples and that they are qualified to take surface samples can be difficult to achieve. Observational assessments have their limits and provide no guarantee of consistency between personnel.
A comprehensive study was performed to understand key contributors to surface sampling variation in the real world. This was accomplished by evaluating surface sampling performance, including microbial recovery efficiency and aseptic technique, of a large population of people in the field using standardized test surfaces. The test surfaces included a precise and stable quantity of viable microbes coated evenly over a test sampling area that was approximately the area of a standard contact plate. These test surfaces were then shipped to sampling personnel across the United States. A dataset of >1,100 sampling events across more than 300 test sites was generated and revealed inconsistent microbial recovery and lapses in aseptic technique (see Figure 2) across the country. For recovery efficiency, 22% of sampling events fell below 20% recovery of viable microorganisms from the surface. For aseptic technique, the data reveal that 8.3% of the sampling events resulted in inadvertent contamination of the contact plates during the sampling operation.
Personnel performance was shown to significantly impact sample recovery, with variability in technique (e.g., pressure, contact time, and rolling technique) emerging as a key factor behind inconsistent results. By assessing both microbial recovery efficiency and aseptic technique, the study underscores the pressing need for standardized training and competency evaluations to ensure reliable and consistent sampling practices.
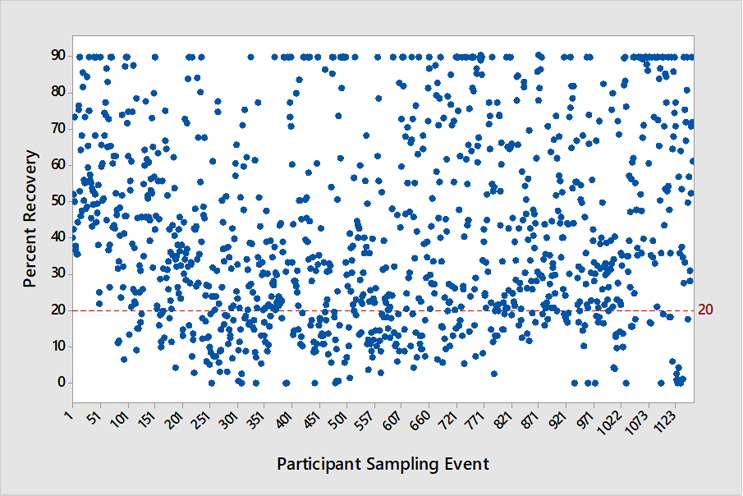
Figure 1. Contact Plate Percent Recovery by Participant Sampling Event. This chart shows the percentage of recoveries by ‘participant sampling event’ for this study (n = 1155). Each data point is the average of 3 microbial-coated test surface replicates. The red line at 20% recovery indicates a recovery efficiency PASS/FAIL threshold that was established based on earlier datasets and contact plate literature.
Conclusion
For sterile compounding and cGMP-regulated facilities, a reliable viable EM program, driven by consistent sampling techniques and qualified personnel, is vital to maintain regulatory compliance, product quality, and patient safety. The data from the real-world viable surface sampling studies, along with evolving regulations, underscore the importance of training and competency of the personnel responsible for EM and the need to ensure that surface sampling is accurate, reproducible, and effective in maintaining a low- or contamination-free environment. Implementing a viable surface sampling competency assessment into annual EM training programs is an important step to ensuring the quality of data generated by EM staff. By prioritizing consistent sampling techniques and qualified personnel, facilities can proactively address contamination risks, help ensure regulatory compliance and ultimately protect patient safety.
References:
- Parenteral Drug Association. Technical Report No. 13 (Revised). Fundamentals of an Environmental Monitoring Program. 2022.
- General Chapter: USP. Pharmaceutical Compounding – Sterile Preparations <797>. In: USP-NF. Rockville, MD: USP; May 01, 2024.
- EU GMP Annex 1. Volume 4. Brussels, 22.8.2022. C(2022) 5938.
- General Chapter: USP. Microbiological Control and Monitoring of Aseptic Processing Environments <1116>. In: USP-NF. Rockville, MD: USP; Official prior to 2013.
 About The Author:
About The Author:
Josh Erickson is a senior director of microbiology pipeline and operations at United States Pharmacopeia. Previously, he cofounded Stratix Labs, which was acquired by USP in 2024. He holds multiple patents, and his career’s focus has been on solving microbiology issues in laboratory, medical device manufacturing, and pharmaceutical settings.
