EntreMed Inc. Forms TheraMed Inc. to Develop Cell Permeation for Drug, Gene Delivery

TheraMed has initiated aggressive timetables for moving is early and late-stage preclinical programs through the development pipeline, a strategy that should be helped by a cutting-edge delivery system. TheraMed will finance its growth through independent financing and strategic partnerships.
TheraMed has four programs currently in various stages of preclinical development, including a flow electroporation device that processes whole blood, separates its components, and inserts biologically active molecules in the cell population of interest. Its closest drug to clinic incorporates inositol hexaphosphate (IHP) into red blood cells to increase tissue oxygen delivery. Other programs include a targeted drug delivery platform through platelets, methods of storing platelets, and targeted gene expression through white blood cells. The potential markets for these product candidates include cancer, cardiovascular disease, thrombosis, inflammatory conditions, and pulmonary disease.
Joining Doerfler on TheraMed's management team will be Joseph C. Fratantoni as VP of Medical Affairs and Clinical Development. Fratantoni previously served as chief for the Laboratory of Cellular Hematology, within the Center for Biologics Evaluation and Research (CBER), at the U.S. Food and Drug Administration.
Shawn J. Green was appointed VP of Research and Vininder Singh will direct instrumentation development. Green currently serves as VP of Discovery Research for EntreMed, and will remain in that position. John W. Holaday, chairman, president, and CEO of EntreMed, will additionally serve as chairman of TheraMed.
Opening Up Red Blood Cells
Cell permeation by flow electroporation uses short duration electrical pulses to open microscopic pores in blood cells, allowing biologically active molecules to diffuse into the cell and its nucleus from outside the cell membrane. These temporary pores then reseal within milliseconds. Electroporated blood cells could be armed with drugs or genes that target sites in the body such as tumors, blood clots, and diseased organs. According to TheraMed CEO Doerfler, life science laboratories worldwide use static electroporation as a standard procedure, "but are greatly limited by the small volumes of sample they are able to process. Usually, they are restricted to the microliter range. By contrast, TheraMed's flow electroporation technology processes cells in quantities of 100 milliliters and above, volumes that may provide meaningful clinical utility."
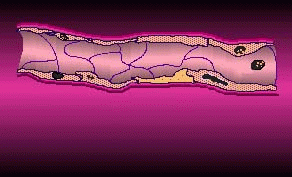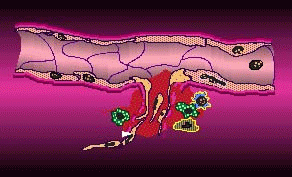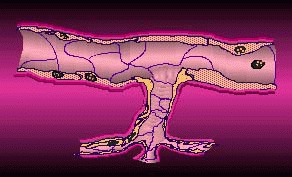
By passing cell permeation onto its subsidiary, EntreMed can concentrate on anti-angiogenesis therapies, which put the company on the map 15 months ago.
EntreMed originally licensed its cell permeation technology from Harvard's Center for Blood Research in 1992. Since then the company ironed out several technical difficulties and identified potential clinical uses for the technology. With those technical difficulties behind it, EntreMed can now re-dedicate itself as a leading angiogenesis drug discovery company while exploiting cell permeation technology through its new subsidiary.
For more information: Douglas A. Doerfler, President, TheraMed, 9640 Medical Center Dr., Rockville, MD 20850. Tel: 301-217-9858. Fax: 301-315-2437.
By Angelo DePalma
