5 Essential Factors For A Successful Biopharma Product Launch
By Christian Seiffert, Peter Rosenorn, and Sej Brar, L.E.K. Consulting

Rapid changes in the biopharma industry are making it increasingly difficult to launch new products successfully. Especially challenging are novel treatment modalities such as gene and cell therapy; increased use of biomarkers; rare diseases with patients that are hard to identify, diagnose, and access; and expanded requirements to demonstrate economic value.
In addition, biopharma companies must navigate declining access to physicians, digitalization, more informed and demanding patients, intense competition, and the need to launch globally to maximize revenues. Shareholders’ expectations are also increasing, driven in part by the high prices paid to acquire the limited number of attractive assets available.
Faced with such challenges, biopharma companies must optimize their launch planning and execution process to ensure successful launches of strong products.
In this article, we review the five key levers for biopharma launch success (see Figure 1) and explain how they inform the creation of a high-performing product launch framework.
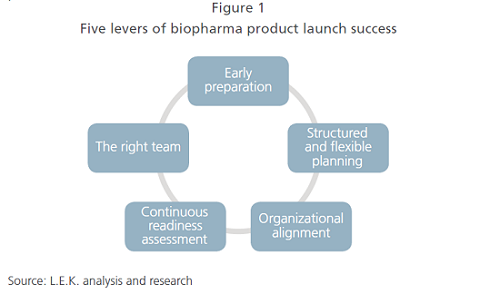
1. Early Preparation
A successful launch requires evidence generation for reimbursement, the identification and development of a new set of advocates, and/or the identification of a suitable patient group. The planning process should start approximately three years before launch, though this may not always be possible if an asset is acquired or in-licensed or if there are budget constraints. However, starting late will most likely lead to missing or lower-quality deliverables, regardless of how many resources are invested, thereby resulting in either a delayed launch or poor launch performance.
When launch planning, the first step is to conduct a thorough situation assessment to determine the unique aspects of the launch, asking questions such as:
- How large is the patient population?
- Are patients easy to identify and diagnose?
- Will a biomarker be needed?
- How many healthcare providers do we need to call on?
- How differentiated is the product?
- How much variability is there in competitive dynamics across key markets?
During this initial assessment, the core launch team, led by the brand or launch lead, identifies priorities, assembles the global launch team, defines timelines, and establishes the launch readiness assessment process. This ensures proper focus on the critical success factors and helps the launch team to define timelines and milestones.
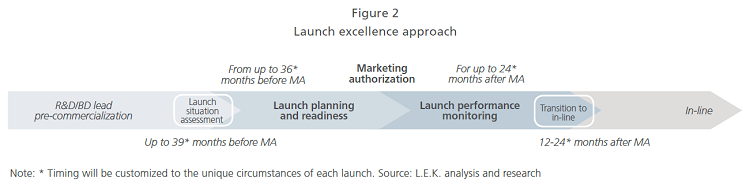
In addition to the early start, biopharmas should implement a well-defined launch excellence approach that guides the process along a clear path from situation assessment to planning and readiness, through to performance monitoring until the brand transitions to “in-line” (see Figure 2 that illustrates both preparation time frames and key planning phases).
2. Structured And Flexible Planning
Launch planning tools are often not adopted because they are too complicated and inflexible. To be useful, the planning process must help the launch team identify and focus on only those deliverables that support the specific success factors.
The launch excellence framework depicted in Figure 3 has three main dimensions (market, product, and organization), which cover 12 strategic launch themes with a total of 50 deliverables.
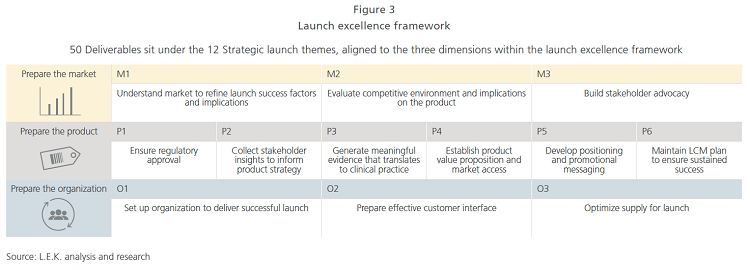
During the initial situation assessment, the core launch team uses market and product information (e.g., product forecasts, competitive dynamics, key product dimensions, R&D/BD timelines) to determine the success factors that will drive the product in the chosen market, as well as the overall launch timeline. Based on the success factors identified, this team can select critical deliverables and map them to the defined timeline, adding owners and expected levels of completion that will later help establish the launch readiness assessment process.
3. Organizational Alignment
A successful launch team needs to be cross-functional, with a clear division of responsibilities between global and local representatives.
Typically led by the product vice president or brand head, the global launch team must have representation from key functions and affiliates; many fail to include enough functional expertise or the input of key personnel. Alongside undertaking the launch situation assessment, the global team is also responsible to ensure deliverables are met and intervene when needed to address locally arising issues.
Affiliate launch teams are responsible for planning and executing the launch locally. They are often led by general managers, business unit heads, or marketing leaders, and they should comprise cross-functional members.
At a minimum, the functions across both teams must include R&D/medical affairs, marketing, market insights, market access, health economics and outcomes research, and regulatory. Depending on the situation, other functions may be required, such as manufacturing/supply chain, finance, HR, compliance, and public affairs.
Finally, genuine two-way dialogue between the global and local teams is an integral part of the launch process. Affiliates must be empowered to ask for support and global representatives must have realistic expectations about what individual affiliates can deliver, especially if there is limited time to prepare.
To support the collaboration, we recommend that the team adopt a web-based, easy-to-use planning and tracking tool to provide transparency and promote accountability (see Figure 4).
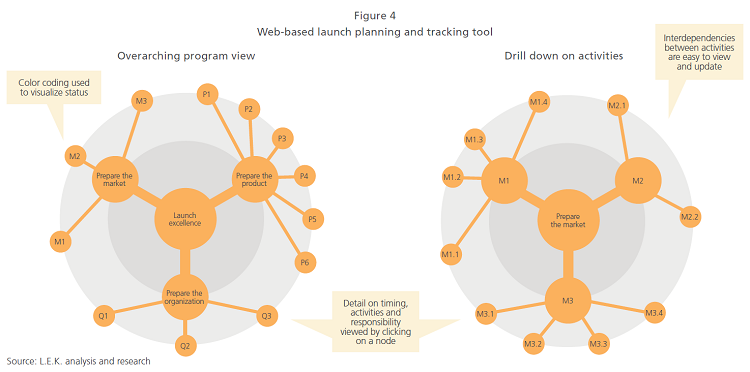
This cross-functional global and affiliate team approach needs to continue throughout the readiness phase and after the launch is completed, though the participation of some functions may be reduced over time.
4. Continuous Readiness Assessments
To help participating countries prepare for launch and facilitate collaboration with global representatives, the team should conduct regular in-depth readiness reviews around key milestones such as the decision to file, filing, and marketing authorization. Additional reviews should be scheduled as necessary (see Figure 5).
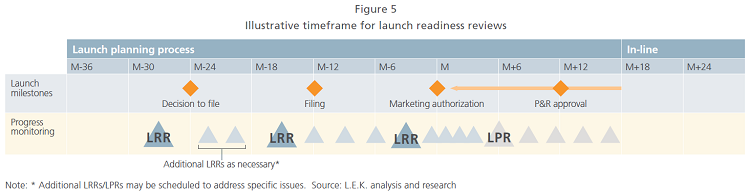
The initial launch situation assessment defines the priorities and timeline of readiness reviews and sets expectations regarding the progress of critical deliverables at any given point in the launch process. Importantly, the readiness reviews should not be “tick the box” audits, but rather high-value sessions that ensure overall alignment regarding progress and next steps. As such, they are integral to launch success.
Existing launch tools, such as the one illustrated in Figure 4, can help monitor readiness and provide input for qualitative discussion at reviews, and these tools should also be used to ensure delays are addressed through actions defined by the launch teams.
5. The Right Team
Regardless of the product, the process, and the launch planning tools, having the right team in place to properly plan and execute the launch is critical to its success. Without the appropriate experience, passion, and collaborative mind-set at both the global and local levels, the launch is unlikely to succeed.
For biopharmas with limited previous experience, this can be particularly challenging, and it is even more important for these companies to start launch preparation early to allow time to hire and/or train new team members. The complexity of a product launch means that the team must include some members with prior launch experience. This will help to identify problems faster, build cross-functional and organizational consensus, sustain motivation and morale, and reinforce the importance of the launch to executive leadership.
Preparing For Launch Success
Any biopharma company preparing to launch should consider eight key questions to determine whether it is on track with planning and execution:
1. Do we understand the key market and product dynamics for our launch?
2. Have we identified the critical launch success factors?
3. Are we focusing our efforts on these factors?
4. Do we have a strong cross-functional global team with representation from the most important affiliates?
5. Have we identified and do we track launch readiness indicators specific to our launch?
6. Do we know what launch success looks like?
7. Do we have a plan to monitor launch performance?
8. Will the organization be prepared to launch our product successfully?
The biopharma industry is evolving quickly and launching products is more challenging than ever. By considering the key levers outlined in this article, biopharma companies will find themselves able to execute their launches in a more targeted and effective way and increase their chances of success.
About The Authors:
 Christian Seiffert is a partner in L.E.K.’s European Biopharmaceuticals and Life Sciences practice. He has more than 17 years of consulting experience, providing strategic advice to leading pharmaceutical, biotechnology, and medical technology organizations. Seiffert has advised clients on a wide range of assignments including commercial excellence, product launch, brand management, pricing and market access, and corporate strategy.
Christian Seiffert is a partner in L.E.K.’s European Biopharmaceuticals and Life Sciences practice. He has more than 17 years of consulting experience, providing strategic advice to leading pharmaceutical, biotechnology, and medical technology organizations. Seiffert has advised clients on a wide range of assignments including commercial excellence, product launch, brand management, pricing and market access, and corporate strategy.
 Peter Rosenorn is a managing director and partner in L.E.K. Consulting’s Boston office. He joined L.E.K. in 2010 and specializes in the life sciences and pharma sector, with a focus on growth strategy and organization and performance. Rosenorn advises clients on a range of critical business issues including organizational scale-up and development, launch planning and commercialization, transaction support, forecasting and valuation, and post-merger integration.
Peter Rosenorn is a managing director and partner in L.E.K. Consulting’s Boston office. He joined L.E.K. in 2010 and specializes in the life sciences and pharma sector, with a focus on growth strategy and organization and performance. Rosenorn advises clients on a range of critical business issues including organizational scale-up and development, launch planning and commercialization, transaction support, forecasting and valuation, and post-merger integration.
 Sej Brar is a partner in L.E.K.’s European Life Sciences practice and is based in London. He has over a decade of consulting experience and has worked on a range of strategic assignments including franchise/product strategy, business development/transaction support, launch excellence, and customer engagement models. He has a particular focus on organization and performance (O&P) within life sciences and holds an MBA (Distinction) from Harvard Business School.
Sej Brar is a partner in L.E.K.’s European Life Sciences practice and is based in London. He has over a decade of consulting experience and has worked on a range of strategic assignments including franchise/product strategy, business development/transaction support, launch excellence, and customer engagement models. He has a particular focus on organization and performance (O&P) within life sciences and holds an MBA (Distinction) from Harvard Business School.
