3 Essentials For A Fit-For-Purpose Compliance Strategy In Decentralized Pharma
By Penelope Przekop, MSQA , RQA P-GCP, and Valerie Przekop
 Outsourcing and technology have morphed our industry into a new decentralized model. Now, each sponsor must carefully develop an ongoing compliance strategy considering its unique business model and its influence on the drug development journey. Strategies are needed to design quality management systems (QMSs) that can expand and pivot in tandem with corporate and clinical development.
Outsourcing and technology have morphed our industry into a new decentralized model. Now, each sponsor must carefully develop an ongoing compliance strategy considering its unique business model and its influence on the drug development journey. Strategies are needed to design quality management systems (QMSs) that can expand and pivot in tandem with corporate and clinical development.
Below, we discuss three essentials for designing a compliance strategy to ensure patient safety and data integrity throughout the decentralized drug development process. An effective compliance strategy focuses on the sponsor’s internal roles, responsibilities, processes, and activities, of which the majority now include working with CDMOs and others in the supply chain.
(Note that a sponsor’s QMS should encompass GLP, GMP, GCP, GPV, and CSV [GxP] activities. The examples throughout this article are not all-inclusive and are intended to relay concepts.)
Essential #1: Understand & Embrace The Fundamental Drivers Of A Successful Compliance Strategy
A successful sponsor-specific strategy can be created and executed when the following four guardrails remain in place. They establish a framework for creativity, innovation, and good old common sense, within our highly regulated reality.
- Expertise: A sponsor’s employees must be qualified to perform their respective tasks. This includes the employees’ ability to oversee their CDMOs from both a compliance and operational perspective (sponsors cannot rely on vendors to compensate for their lack of expertise).
- Regulation: A sponsor must comply with all applicable global health authority regulations. A company cannot create a fit-for-purpose quality system based on the science of quality management unless key leaders understand and respect the industry and its requirements.
- Risk: Strategic decisions for QMS design and execution must include the assessment of potential risk to patient safety and data integrity. Sponsors remain responsible to oversee risk identification, assessment, and mitigation, including potential risks introduced by engaging multiple CDMOs.
- Documentation: If it isn’t documented, it didn’t happen. If you aren’t documenting it, who is? Your CDMO? Many sponsors have learned the hard way not to assume that CDMOs are appropriately creating and retaining the documentation required to follow all applicable regulatory requirements and standards.
Essential #2: Understand How To Define Sponsor Complexity
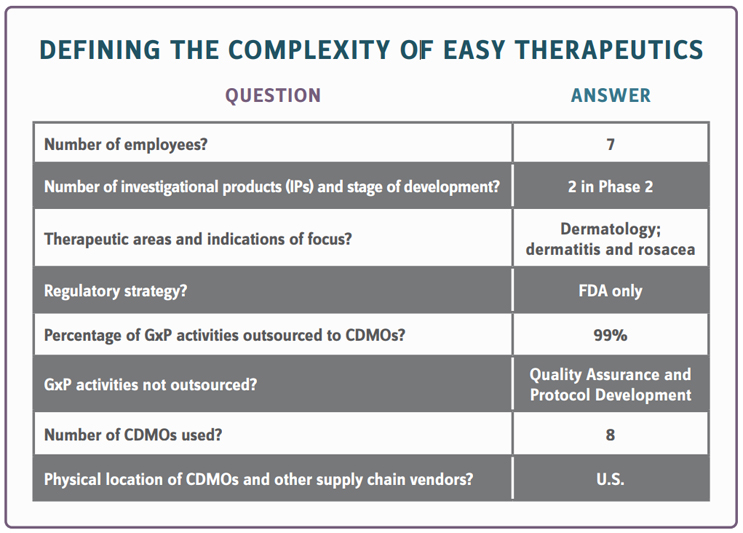
Sponsors must clearly define their current level of complexity to create an appropriate compliance strategy. This complexity may shift and skyrockets along with corporate and clinical pipeline development; therefore, defining it is an ongoing activity. Maintaining a flexible QMS is crucial to compliance strategy design. The elements that define sponsor complexity and inform the design of a fit-for-purpose QMS fall into two categories: business model and product development pipeline.
The information below provides a conceptual understanding of how a sponsor, in this case, the imaginary Easy Therapeutics (ET), can begin to define the complexity involved in its mission to execute a successful drug development program.
Essential #3. Understand The Connection Between Sponsor Complexity & Compliance Strategy
If you’re wondering how the information above relates to compliance strategy or a QMS, you’re not alone. Many experienced pharma professionals don’t intuitively recognize the connection, simply because the science of quality management, pharma quality, and compliance are not their areas of expertise. To gain a conceptual understanding of how sponsor complexity can guide development of a fit-for-purpose compliance strategy within the guardrail framework described above, consider the following examples.
QMS Process Example 1: Development and Maintenance of Controlled Procedural Documents
Required: Regardless of company attributes, ET is required to document its GxP processes. Outsourcing a GxP activity does not exclude ET from participating in the activities; it only changes the focus and actions it will take.
ET Strategic Considerations: ET requires a basic process for how they document and control their GxP processes. Due to heavy outsourcing to CDMOs, the ET list of documented processes should include the GxP activities that ET executes in-house, how they qualify and oversee CDMOs, develop protocols throughout the drug development process, and manage the other QMS elements required. How ET develops and maintains controlled procedural documents can be straightforward, given they only have seven employees. They can operate without an electronic document management system provided they ensure controls are in place to meet regulatory requirements. The challenge is doing this the right way, particularly if none of their seven employees have expertise in quality systems.
QMS Process Example 2: Vendor Qualification
Required: If a sponsor uses one or more CDMOs, this QMS element is required. The number of CDMOs, their locations, contracted activities, etc., drive the complexity of several QMS elements, including this one. Regulators require GxP vendors to be certified by a qualified individual who is independent of the operational team. If there is a risk-based reason why a sponsor decides that a CDMO does not require qualification, it must be fully documented and defendable to a regulatory inspector.
ET Strategic Considerations: ET requires a robust process for CDMO qualification. The process should be detailed enough to ensure the employees involved understand roles and responsibilities. It’s critical that they monitor corporate and drug development progress to expand and deepen the CDMO qualification program as needed. How to document decisions not to qualify a CDMO also should be part of the strategy.
QMS Process Example 3: Quality Assurance Auditing
Required: A basic process should be established, not only for auditing CDMOs, but also for internal processes, clinical sites, databases, documents, trial master files, etc., if applicable.
ET Strategic Considerations: How auditing will happen is the strategic decision. If auditing is outsourced, an internal process is still required. Again, the strategic approach dictates the internal process; it does not make it go away. ET is required to be involved and therefore must document that involvement. This could include selection and oversight of contracted auditors as well as how audit documentation flows between all parties, where it’s retained, etc. If the strategy is to outsource all audits, the next decision is not, “Therefore, we don’t need an internal process.” ET is required to document how auditing of GxP activities takes place, and then to make it happen.
A more complex sponsor profile, whether related to having a more robust pipeline, a mix of outsourced and in-house GxP activities, electronic systems, and far more employees, increases the complexity that must be addressed in designing a fit-for-purpose compliance strategy and QMS. This doesn’t mean that the QMS must be complex. Einstein said, “The definition of genius is taking the complex and making it simple.” Simple solutions can be created. The industry is challenged to rethink how to ensure products developed in a decentralized, and often disjointed, drug development process continues to be safe and effective. The concepts discussed above can serve as a basis for innovative changes to GxP quality systems processes that have been overlooked despite their primary purpose of ensuring data integrity and patient safety. It’s not all about outsourcing; it’s about how outsourcing can best work as we continue our shared mission to bring new healthcare solutions to market and ensure public health.
 Penelope Przekop is a corporate quality management expert. Throughout her 30+ year career, she has worked with numerous Fortune 100 pharma companies, including Pfizer, Merck, Lilly, and Glaxo Smith Kline, and held leadership positions at Novartis, Covance, Wyeth, and Johnson & Johnson. She is the founder and CEO of PDC Pharma Strategy and serves as the Chief Compliance Officer for Engrail Therapeutics. She is the author of Six Sigma for Business Excellence (McGraw-Hill). Przekop earned a BS in biology from Louisiana State University and an MS in quality systems engineering from Kennesaw State University. She is a graduate of the Smith College Program for Women’s Leadership and the Rutgers University Senior Leadership Program for Professional Women. Her new book, 5-Star Career: Define and Build Yours Using the Science of Quality Management launches in November 2021 from Productivity Press.
Penelope Przekop is a corporate quality management expert. Throughout her 30+ year career, she has worked with numerous Fortune 100 pharma companies, including Pfizer, Merck, Lilly, and Glaxo Smith Kline, and held leadership positions at Novartis, Covance, Wyeth, and Johnson & Johnson. She is the founder and CEO of PDC Pharma Strategy and serves as the Chief Compliance Officer for Engrail Therapeutics. She is the author of Six Sigma for Business Excellence (McGraw-Hill). Przekop earned a BS in biology from Louisiana State University and an MS in quality systems engineering from Kennesaw State University. She is a graduate of the Smith College Program for Women’s Leadership and the Rutgers University Senior Leadership Program for Professional Women. Her new book, 5-Star Career: Define and Build Yours Using the Science of Quality Management launches in November 2021 from Productivity Press.
 Valerie Przekop is a candidate in the Stanford University Class of 2022 in the areas of M.S., biomedical informatics; B.S., management science and engineering with a concentration in operations and analytics; and B.A., psychology. As the associate director of product development for PDC Pharma Strategy, Valerie leads the development of innovative quality and compliance-related products, and provides GxP quality assurance and compliance consulting services to pharma and biotech companies.
Valerie Przekop is a candidate in the Stanford University Class of 2022 in the areas of M.S., biomedical informatics; B.S., management science and engineering with a concentration in operations and analytics; and B.A., psychology. As the associate director of product development for PDC Pharma Strategy, Valerie leads the development of innovative quality and compliance-related products, and provides GxP quality assurance and compliance consulting services to pharma and biotech companies.
