Esteve Quimica's New Site Receives FDA Full Clearance To Commercialize Products In US
LIÇÀ DE VALL – Esteve Quimica’s new site receives FDA full clearance to commercialize products in US.
The acquisition of a new industrial site in Lliçà de Vall (Barcelona) early in 2022 increased Esteve Quimica’s active pharmaceutical ingredients production capacity by 20%.
The operation was an excellent opportunity to continue positioning Esteve Química as an international reference partner for the development and manufacture of active pharmaceuticals ingredients for innovator pharma companies around the world.
At the time of the acquisition, Lliçà de Vall plant hold a Good Manufacturing Practices certificate, issued by AEMPS (Spanish Health Agency) and Environmental ISO14001:2005 certification. However, the API exports to US were under an import alert.
Thanks to an extraordinary leadership, change management and collaborative work to implement Esteve Quality Management System (QMS), while continuing to manufacture high-need APIs, Esteve’s new site has recently been granted by FDA a full clearance to commercialize API in USA.
This landmark accomplishment, evidenced also by the several successful customer audits, represents Esteve Química’s way of working and demonstrate the commitment with Quality, Safety Health and Environment. Additionally, a new ISO 45001 on Health and Safety certification has been recently granted to LLiçà de Vall site as well.
This journey counted with Esteve Química’s best-in-class team, who worked restlessly to revamp the new site in record time, only 12 months!
Esteve Química’s is true to its purpose that is to serve customers and patients worldwide to improve people's health.
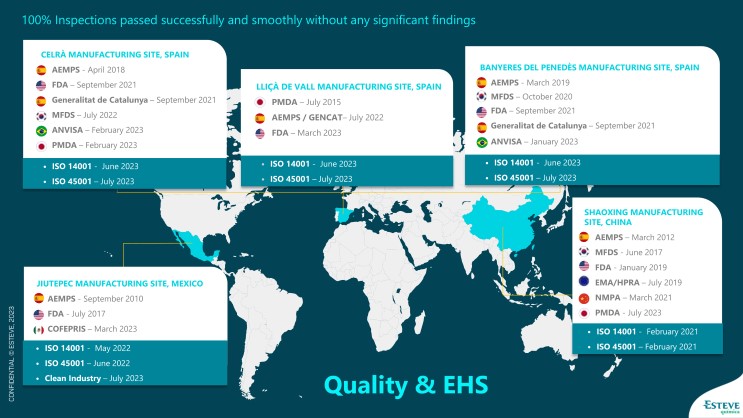
For full access to Esteve Química’s Health Authority site inspections history, click the image below to enlarge: