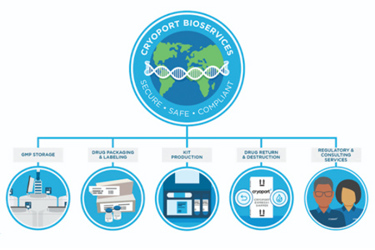
Global BioServices: Secondary Packaging And Labeling Solutions
Cryoport Systems’ highly anticipated Global Supply Chain Centers have been operational for a few months, and customers continue to move biopharmaceutical materials into our Houston, Texas and Morris Plains, New Jersey facilities in order to mitigate risks.
Our new Global Supply Chain Centers were established in direct response to client requests, and represent the initial stages of a global network that offers specialized logistics support and BioServices solutions. Therapy developers require dependable partners with suitable facilities to help them manage their valuable materials, such as therapeutic products and manufacturing raw materials.
In addition to materials storage, Cryoport Systems’ facilities were designed to provide specialized drug packaging, labeling, and custom kit production – a set of services we refer to collectively as BioServices. Secondary packaging is typically required for cell and gene therapy products to protect fragile cryogenic and ultra-cold primary containers. Our Bioservices team can provide secondary packaging and labeling solutions for vials, bags, and other containers, which are customized to meet the study protocol and clinic identification requirements.
The packaging and labeling processes are performed at the optimal temperature, ensuring that the product does not experience a temperature excursion during the preparation for shipping. We also offer assistance with label text design, translations, blinding, and regulatory support.
Application methods and container types can have a substantial effect on long-term adhesion, and labels must be able to withstand both cryogenic storage temperatures and rapid warming during the thawing process. Due to the complexity of these medications and the limited options for over-labeling once the materials have been frozen, it is vital to choose the proper label the first time, and to ensure the solution is suitably validated – this is another service we can provide.
To provide the capacity and services required by our expanding clientele and the cell and gene therapy industry, we have integrated innovative technologies, automation, and streamlined operational procedures into our facilities. Cryoport Systems' BioServices are part of the next phase in facilitating the global distribution of life-saving medications and validated secondary packaging.
