Helping Pharma Manufacturers Overcome DSCSA Interoperability Pain Points
By Mike Karhoff and Mark Karhoff, Ten Count Consulting, LLC
With the FDA guidance documents and requirements of the DSCSA (Drug Supply Chain Security Act) still unfolding,  there is a growing need for sectors in the supply chain to align on how to meet various aspects of the law. We believe 2020 and 2021 will be critical years in determining if the industry can align on how to build an interoperable system that meets the basic requirements that should be implemented in 2022 and 2023. DSCSA, by design, is vague in the later phases leading up to the 2023 requirements of interoperability. While this provides the ability for those who understand the businesses to help shape the systems and processes, it also leaves room for confusion and inefficiencies where there is misalignment, which may ultimately make true interoperability impossible and limit the ability to further protect patients.
there is a growing need for sectors in the supply chain to align on how to meet various aspects of the law. We believe 2020 and 2021 will be critical years in determining if the industry can align on how to build an interoperable system that meets the basic requirements that should be implemented in 2022 and 2023. DSCSA, by design, is vague in the later phases leading up to the 2023 requirements of interoperability. While this provides the ability for those who understand the businesses to help shape the systems and processes, it also leaves room for confusion and inefficiencies where there is misalignment, which may ultimately make true interoperability impossible and limit the ability to further protect patients.
Many leading organizations in each DSCSA named sector (manufacturer, repackager, distributor, dispenser, and third party logistics [3PL]) have been working diligently over the past years to bring alignment and needed standards into reality. While efforts across the industry have been active in groups like GS1 and the newly formed PDG (Partnership for DSCSA Governance), some sectors have been able to align more than others in areas that require sector specific consensus. The HDA (Healthcare Distribution Alliance) has done significant work with the distributor community on compliance approaches for 2019 and 2020 while also working in areas of integration with manufacturers and dispensers. However, we believe that manufacturers and dispensers need similar forums for sharing compliance approaches through industry groups or risk the development of variations that could make compliance substantially more costly and difficult.
The Pilot Approach
These misalignments have started to move more to the front as DSCSA unfolds, as manufacturers are often finding that decisions on how to meet compliance requirements can vary among manufacturers, which causes conflicting business requirements for solutions providers and integrations with other sectors.
Ten Count Consulting conducted a benchmarking pilot activity with four leading pharmaceutical manufacturers during the second half of 2019 to provide an open forum to discuss DSCSA requirements. Our objectives in the effort were to:
- Understand three key areas needing further industry discussion on DSCSA
- Develop, conduct, and improve a process for building best practices
- Identify findings and next steps for each of the topics.
We facilitated this activity, under antitrust guidelines, by holding meetings every few weeks during late 2019, with follow up activities and surveys of participants. Our initial activity revolved around priority topic selection.
The steps developed to conduct the pilot included:
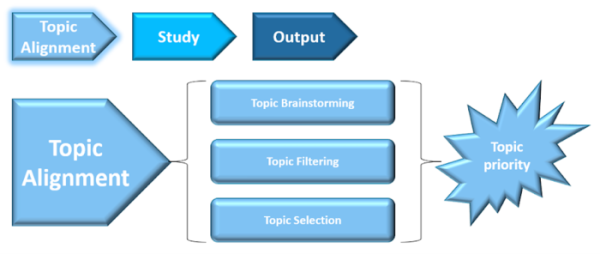
As a result of these initial steps, the group aligned on three topics that were the focus of our pilot activities:
- Requirements for authorized trading partner checks
- Returns product verification message exception handling
- Verification enforcement discretion impact and planning
These topics were focused on identifying the requirements and building outputs that could be utilized in cross-sector alignment discussions. The intent was to discuss positions on each topic so the group could ensure each company had a broader perspective of factors to consider in their respective approach to each topic.
In subsequent meetings, the group worked through each topic by collecting each company's position and, where needed, conducting surveys or brainstorming with pros and cons of each area. We then refined these positions by working the gathered requirements down to a consolidated list and identifying outputs for each topic.
The resulting outputs of the three topics were shared with the group and through a manufacturers’ best practice LinkedIn group that is open to any interested manufacturer. This approach allows manufacturers to understand how other manufacturers are approaching topics to help better prepare them to work with other sectors through cross industry and standards workgroups that operate on a regular basis. The outputs for each topic are highlighted here:
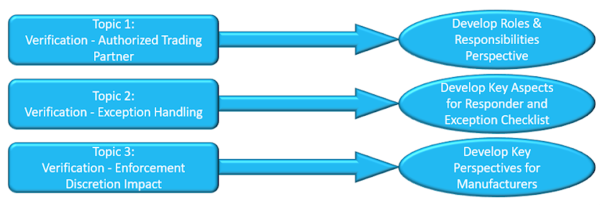
We concluded activities by capturing next steps for manufacturers to consider as priorities in 2020:
- Focus on sector alignment on best practices to meet DSCSA compliance through 2023
- Reduce risk of patient safety due to conflicting positions and approaches that ultimately make interoperability harder
- Eliminate lost time and cost of working out issues in industrywide groups
- Prepare for increased complexity as interoperability requires alignment on key principles
- Utilize the PDG and GS1 workgroups on DSCSA to understand cross-sector requirements and capture topics for alignment of each sector
Conclusions
We believe there is a need within each pharma sector for more constructive discussions of differing perspectives on how to best address compliance requirements. We encourage members of all sectors impacted by DSCSA to discuss this need with their company leadership, legal counsel, industry trade groups, and peers. Be sure to highlight examples of areas where your sector may need to reduce risk created by various interpretations of the law. As you work with industry groups, be sure to understand how the discussions can support overall industry interoperability in groups such as PDG as well as standards groups such as GS1.
We would like to thank the pilot participants for their time and inputs. Our hope is that a manufacturer trade organization will take advantage of the opportunity to help the industry align better on patient safety and consider coordinating similar workgroups as needed to help avoid diversity of interpretations of the law that would cause significant work for the entire industry. We are happy to discuss this approach with any trade organization and willing to share the process and topics collected in the interest of a more secure U.S. drug supply chain.
About The Authors:
 Mike Karhoff is a supply chain and project management consultant at Ten Count Consulting, LLC. He serves as the DSCSA assessment lead and coordinates a pharma manufacturer best practices forum. Mike has over 25 years of experience in project management, manufacturing quality, and engineering roles. He has a diverse background of project management in the pharma, foundry, and automotive industries. He lives with his family in Northwest Ohio and travels to clients throughout the U.S. You can reach him at mike.karhoff@tencountconsulting.com or connect with him on LinkedIn.
Mike Karhoff is a supply chain and project management consultant at Ten Count Consulting, LLC. He serves as the DSCSA assessment lead and coordinates a pharma manufacturer best practices forum. Mike has over 25 years of experience in project management, manufacturing quality, and engineering roles. He has a diverse background of project management in the pharma, foundry, and automotive industries. He lives with his family in Northwest Ohio and travels to clients throughout the U.S. You can reach him at mike.karhoff@tencountconsulting.com or connect with him on LinkedIn.
 Mark Karhoff is a supply chain consultant and founder of Ten Count Consulting, LLC. He has 25 years of experience in program management, process improvement, supply chain solutions, and blockchain, as well as DSCSA (Drug Supply Chain Security Act) compliance. Mark has served as a contracted pharmaceutical client representative with industry groups such as PDG (Partnership for DSCSA Governance), HDA, GS1, and PDSA to establish standards and build alignment on solutions to meet the unfolding U.S. law. He lives with his family in Chicago and believes in advancing corporate social responsibility through his volunteer work with a job skills program for 18- to 24-year-old urban youth called Year Up-Chicago. You can reach him at mark.karhoff@tencountconsulting.com or connect with him on LinkedIn.
Mark Karhoff is a supply chain consultant and founder of Ten Count Consulting, LLC. He has 25 years of experience in program management, process improvement, supply chain solutions, and blockchain, as well as DSCSA (Drug Supply Chain Security Act) compliance. Mark has served as a contracted pharmaceutical client representative with industry groups such as PDG (Partnership for DSCSA Governance), HDA, GS1, and PDSA to establish standards and build alignment on solutions to meet the unfolding U.S. law. He lives with his family in Chicago and believes in advancing corporate social responsibility through his volunteer work with a job skills program for 18- to 24-year-old urban youth called Year Up-Chicago. You can reach him at mark.karhoff@tencountconsulting.com or connect with him on LinkedIn.
