How An Emerging Biopharma Changed Its Supply Chain Methodology — And What It Learned
A conversation with David Geoghegan and Hal Hunt, Trevena Inc.
When Trevena, Inc. needed to establish a supply chain for its lead compound, oliceridine fumarate — a pain-management product expected to make the transition from clinical trial material to full-blown commercial manufacturing — executives at the emerging biopharma decided to try a new framework. Instead of simply relying on decades of collective experience in managing drug supply chains, they augmented that experience with a new set of tools developed by FDA officials and industry professionals under the Xavier University Supply by Design (SbD) Initiative.
Pharmaceutical Online recently asked two members of Trevena’s management team about their experiences using the SbD tools, and what they learned through the process. David Geoghegan, senior VP of operations, has over 28 years of experience in a variety of operations roles and is currently overseeing Trevena’s third-party supply chain for clinical and commercial supply. Hal Hunt, director of technical operations and CMC supply chain, has over 38 years of experience and is responsible for establishing and managing a third-party (virtual) supply chain to support commercialization of oliceridine fumarate.
How did Trevena get involved with Xavier University and its supply chain tools?
 We were introduced to the SbD project via a “piloting” meeting, which was hosted by Shire and attended by several other interested companies from both the vendor side and the purchasing company side. The meeting was basically a dry run of the tools as they had been developed to that point.
We were introduced to the SbD project via a “piloting” meeting, which was hosted by Shire and attended by several other interested companies from both the vendor side and the purchasing company side. The meeting was basically a dry run of the tools as they had been developed to that point.
We were interested in the tools to see if they could inform our decision making process when evaluating various vendors, as well as help us involve the larger organization to ensure all technical functions were represented. Although we have an experienced team of biopharma professionals, we were looking for a structured approach that might help us reduce time and effort, and improve the quality of our decision making.
2. What are the main components of the SbD process/tools that you implemented at Trevena?
We employed the self-qualification, cross-functional team formation, and initial selection criteria tools over two separate projects.
The first tool we implemented was the cross-functional team formation tool, which forms the foundation of the SbD work. This tool listed the typical functional roles inside a pharma company, what those functional areas can add to the supplier selection process, and what information those areas need from the supplier selection decision. A scoring mechanism guided us through the process of asking each of these functional areas if they feel their involvement in the process had been missed, or if they were not included at the right time. Since Trevena is a small company, many of the functional roles are within the CMC (chemistry, manufacturing, and control) and quality departments, but the tool helped us formalize what each role should contribute to the process. The model that Xavier developed is most effective when the cross-functional team tool is used first, so that the right team is formed to align on the decisions made with and through the other tools.
The self-qualification tool provided an assessment process for the strengths and weaknesses we bring to the supply chain development and management process, such that we could then map those gaps against those of our suppliers. The self-qualification included questions in the following four categories: (1) product and process technical capability, (2) operating systems and business capability, (3) relationship alignment, and (4) quality and regulatory compliance.
An initial selection criteria tool assessment of supplier strengths and weaknesses was then conducted against the same four categories assessed during the self-qualification. The team evaluated potential suppliers’ ability to meet the needs we identified as important. For example, the assessment explores supplier capacity, effective and timely communication, GMP and non-GMP compliance, and demonstrated expertise in specific technical capabilities.
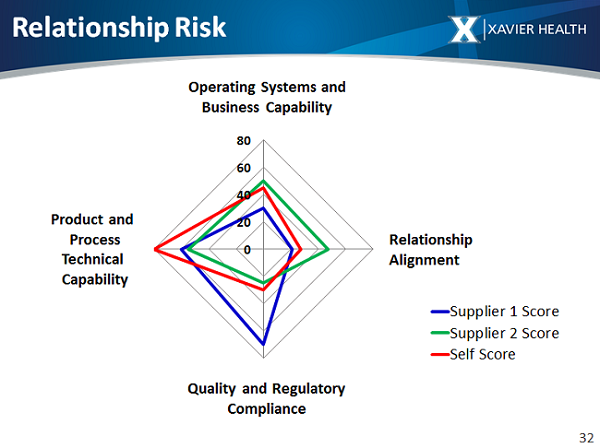
Figure 1: Sample radar chart comparing strengths and weaknesses of suppliers across four risk categories
3. How difficult was it to implement these tools?
The SbD tools were easy to use. Each company must look at how best to incorporate them into their existing framework, systems, and procedures. However, as an emerging company, we were able to use the tools when our commercial system and procedures were in early development. In several areas, we incorporated the concepts into those new system SOPs (standard operating procedures). For established companies that have existing procedures, the tools still may be useful to refresh long-established procedures.
4. How different was the process than other approaches you have used to build out supply chains in the past?
The primary difference is that the tools provided a ready-made roadmap and structure that we lacked. Previously, decisions were made — as they often are in emerging companies — based on the experience of the pharmaceutical development and clinical supply teams.
5. What functional groups were involved in the process at Trevena, and in what capacity?
As we mentioned previously, most of the functions involved in supply chain development and management reside in our CMC and quality departments. However, through the use of the cross-functional tools provided by the SbD process, we recognized that our legal, IT, and commercial operations groups should be included at various points in the supplier selection process to make sure we were aligned on critical attributes needed in a supplier
The existing supply chain was also evaluated with these groups, using the tools. Potential improvements in existing vendor relationships and processes were identified.
6. Based on your analysis, what did you determine were the most important attributes Trevena needed in a supplier?
For us, the three most important supplier attributes were: (1) commitment to quality, (2) sensitivity to costs, and (3) transparency in communication. Obviously, the appropriate technical capability and experience were prerequisites — those attributes were required to land on our short list of finalists. Quality and cost considerations were naturally important (and understood). However, the most important attribute that emerged from our use of the SbD tools was transparency in communication. This is important for success of any contract relationship that may impact our delivering on goals and objectives (e.g., capacity constraints, planning challenges, personnel changes, etc.).
7. Did the process reveal any surprises?
The most dramatic aha! moment for us came while using the self-qualification tool. As an emerging company, we have a small team of very experienced and knowledgeable people who are often asked to perform many functions — some of which are on the edges of their areas of expertise. The discussion of self-qualification really provided a structure to highlight where those “stretches” were occurring. Once we identified them, we were able to look for specific areas of support from internal colleagues or by using our outside networks and selected consultants. Wearing many hats can be a rewarding part of working in emerging biopharma, but it’s important to know the limits. The self-assessment tool provided a useful means of identifying and communicating our needs when managing limited resources.
8. What other lessons did you learn from going through this process?
A key lesson was that the SbD tools could be used as a roadmap for emerging companies to transition from an early clinical supply chain into a more robust Phase 3/commercial supply chain. We were able to use the tools to improve our vendor selection process, which allowed us to establish a robust commercial supply chain for our lead development compound.
9. How did you modify the tools to suit your specific organization and its needs?
We modified the scoring system. For some categories, we used numeric values, but for other categories we used a binary approach — either on or off, rather than a sliding scale. We felt that some categories and/or characteristics were either all or nothing, i.e., they were absolute requirements.
We also had to adjust how we used the tools for the relatively few suppliers we already have as an emerging company, since the tools were designed as a comprehensive set of criteria for larger, more complex supply chains. As an emerging company, we have fewer products and fewer vendors in our supply chain, so we adjusted the use of the tools accordingly.
10. How can readers learn more about and gain access to these free tools?
They can visit the Supply by Design Initiative page on the Xavier University website, and/or contact Marla Phillips, director of the Xavier Health organization at Xavier University, at phillipsm4@xavier.edu or 513-745-3073.
