How Broadening The Analysis Of Compound Factors Allows For Predictive Solubility Solutions
By Matt Wessel, Marshall Crew, Sanjay Konagurthu and Tom Reynolds, Patheon

The Biopharmaceutics Classification System (BCS), developed by the U.S. Food and Drug Administration to simplify and accelerate the drug development process, helps companies when they file for bioequivalence of dosage forms based on in vitro dissolution testing. The objective of the BCS system is to predict in vivo performance of drugs from in vitro measurements of solubility and permeability. The system has evolved to classify low-soluble drugs according to their permeability (BCS Class II or IV). A compound's classification (I through IV) is indicative of its potential bioavailability.
Companies also have adopted the BCS system as a test for a compound's oral delivery, leading decision-makers to believe that knowing a compound's solubility (logS) and lipophilicity (logP) can guide them to the right choice of formulation.
While understandable, relying on these parameters to identify solubility solutions oversimplifies the challenge. A Patheon analysis of drugs brought to market over the past three decades shows that approved drugs do not follow clear trends when these two measures alone are considered.
Additional factors can provide a more complete picture.
Today's molecules require additional criteria
As noted in our recent paper (“How to Choose the Right Solubilization Technology for Your API”), recent trends indicate that logS is decreasing and logP is increasing for new small-molecule medicines. The reason for this shift is two-fold.
First, improvements in synthetic chemistry and high-throughput screening have expanded the small-molecule chemical space that can be accessed. This expansion has led to more novel compounds with desirable potency that present greater solubility challenges. The result: a landscape with more molecules worth investigating that are difficult to make effective in vivo.
The second reason for the shift: More emphasis on compounds that target less “druggable” entities (such as kinases), which typically require more lipophilic compounds to capture potency.
With these two factors in play, there are additional factors to analyze besides logS and logP when considering a solubility solution, including melting points, pKa, permeability, a compound's potency, and dosage levels. Analysis of these factors, in combination with the traditional approach of examining logP and logS, allows us to develop a method for narrowing the range of potential solubility solutions a company should consider.
A look back at drug solubility solutions for three drugs on the market provides a useful illustration.
Similar readings for two properties, but different solubility solutions
A map of compounds as a function of logP and logS, shown in Figure 1, illustrates the limitations of the traditional approach of relying on just these two properties. In the plot, drug products using dispersion and lipid technologies are shown in red and black, respectively. As the figure shows, these two properties alone do not differentiate which technology is appropriate. There is no discernable grouping of the compounds in this particular chemical property space plot.
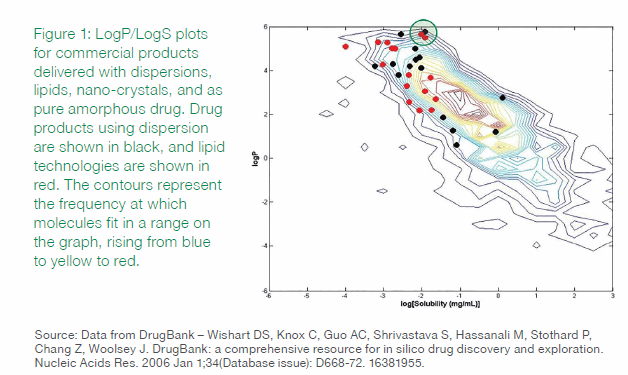
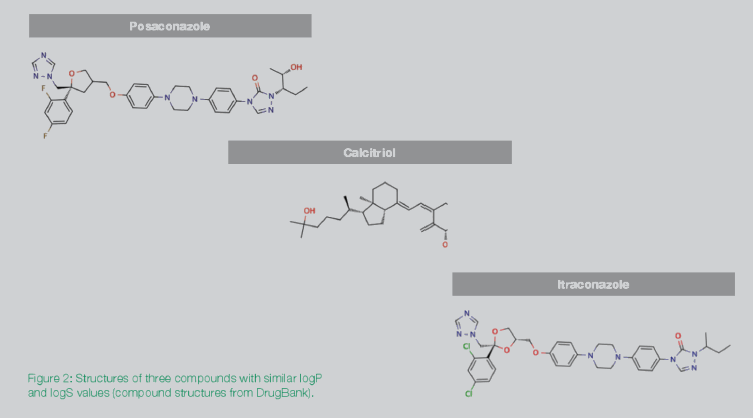
The green circle in Figure 1 surrounds three drugs that use distinct solubilization technologies. But while the three drugs – calcitriol, itraconazole, and posaconazole — essentially have the same logS and logP values, they have different formulation and process development histories. By examining other traits of these compounds, and their histories, we can derive insights into why they each used different solubility technologies. (It is worth noting that itraconazole and posaconazole are structural analogs that target the same enzyme. Calcitriol was developed for a different target enzyme and indication.)
The three structures in Figure 2 are commonly used drug compounds, but for different indications. Calcitriol is notably different in structure from the other two, while itraconazole and posaconazole are clearly analogs of each other. The structural differences between calcitriol and the other two compounds suggest that structure is also a differentiator with respect to technology. However, without looking at the respective structures, the similar logS and logP values for the three compounds can be misleading.
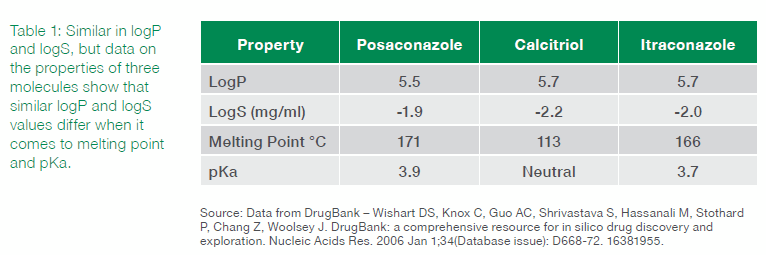
As can be seen in Table 1, there is very little difference in the logS and logP values. However, differences in melting point and pKa are evident. These differences can be used, in part, to drive rational decisions regarding formulation choices. The history of the three compounds bear this out:
Itraconazole uses hot melt extrusion (as Onmel) and spray-layered coated beads (as Sporanox). Itraconazole is an antifungal agent also used for other indications. It comes in two oral dosage forms, Sporanox and Onmel. Sporanox (first marketed in 1999) uses spray layered coated bead technology. The drug, in the form of a solid dispersion with hydroxypropyl methylcellulose (HPMC), is spray layered onto spherical sugar beads to form a solid dispersion.
Spray layering is advantageous in that it can use conventional, solvent-capable fluid bead processing equipment (in contrast to spray drying that requires a specialized process train). A disadvantage with spray-layered beads is that loading them to meet larger unit dose levels can be challenging. In fact, the recommended dosage of Sporanox is one to four 100 mg active capsules per day, depending on the indication.
Approximately six years into the product’s lifetime, it was recognized that given the moderate melting point of 166° C for itraconazole, hot melt extrusion (HME) technology could be used to form a solid dispersion. This dosage form, known as Onmel, uses Meltrex® technology. This has the advantage of allowing a larger unit dose (e.g., 200 mg) to be delivered in a single tablet, improving patient compliance.
Posaconazole uses hot melt extrusion (as Noxafil). Posaconazole, a close structural analog to itraconazole, was recognized as amenable to HME, given its moderate melting point (172° C), and the fact that, as noted above, itraconazole was successfully formulated using HME technology. Early on, Merck (a well-known user of HME technology) evaluated spray drying and HME as dispersion formulation strategies for posaconazole. Spray-dried dispersions showed improved bioavailability, meaning that the HME dispersion form would presumably behave in a similar manner.
It is well known that HME (a "solvent-less" approach) is a less expensive manufacturing process than spray drying, and can be made continuous. Merck also has had a successful history of using HME technology to formulate several of its products. It is reasonable to assume that these two factors led Merck to select HME as its formulation strategy. The final HME dosage form of posaconazole. or Noxafil, contains 100 mg of active drug per tablet, half as much as itraconazole. as posaconazole is more potent against the target enzyme.
Calcitriol uses a lipid solubilization solution. The third compound. calcitriol, has a very different molecular structure than both itraconazole and posaconazole [see Figure 2], despite their similar logP and logs values. Calcitriol exhibits good solubility in lipidic vehicles, and is a soft-gel lipid formulation Rocaltrol. The low-unit dose of Rocaltrol (0.5 µg) does not require large soft-gel capsules, and is another reason why a lipid soft-gel works. The lower melting point of calcitriol (113° C) suggests that other formulation technologies, such as HME and spray drying, also may be suitable.
In the final analysis. three seemingly similar compounds, with nearly identical logP and logS values, are not similar when it comes to the solubilization technologies that apply to them. The differences in melting point, and their effective dosages, are major factors in understanding what solutions are best for each.
ltraconazole and posaconazole are very similar in logP, logS, melting point, and overall structure. However, because of posaconazole's potency, Merck was able to produce a lower-dose tablet. Higher doses limit what solubilization technologies are available. For example, higher doses are difficult to achieve in complexes and coated beads as the amount of available material space is limited, and the tablet size would become too large.
Lower melting points usually mean lower crystal structure lattice energies (although not always), meaning it's easier to break up the lattice. This corresponds to better solubility in liquids. Therefore, it is not surprising that calcitriol works in a lipid formulation. The logP value is also a factor in calcitriol: higher logP values imply better permeability, as well as improved solubility in lipids.
Applying the analysis to other drugs
We can see from this analysis that clear knowledge of first-order properties in addition to logS and logP is necessary to define the best solubilization technology for a given BCS Class II drug with high permeability and low solubility. We have found that the melting point, dose, and permeability, in addition to the logS and logP, directly impact the suitability of the technology.
We have analyzed data about these features across many more compounds, and developed a tool that provides effective solubilization technology choices depending on the therapeutic indication targeted, the type of molecule, the preferred delivery route, and dosage levels. We have validated this algorithm against commercial drugs on the market. And we are using it now to help our clients narrow their technology choices at the start of a project, enabling them to minimize their costs and shorten their timelines.
While it might have been attractive to consider a standard formulation (e.g., crystalline salt form) for Isoptin, Accupril, and Rapumune, a clear and thorough understanding of the molecule’s properties improves a company’s ability to make the right solubilization technology choice early on, leading to faster development timelines. Also, knowing the optimal formulation technology earlier in the discovery process can drive better decisions about which compound for a given project should move forward to pre-clinical development. A robust, scalable, and effective formulation choice in pre-clinical development can help companies avoid the pitfalls of reworking a formulation strategy during Phase 2 or Phase 3 trials.

Narrowing the solubility solution search
In recent years, drug developers evaluating solubility solutions have focused on just two characteristics: The logS of the compound, and its logP.
While knowing logS and logP is clearly necessary, we need to understand and consider additional characteristics of a poorly-soluble compound — including melting points, potency, and dosage levels — to determine the best solubility solution. If all factors are not considered, many wasted cycles of effort can be expended on formulation development. When these efforts lead to a formulation dead end, those costs cannot be recovered. It is better to narrow the choices early, rather than waste time and money by exploring many options, including those that — when other factors are considered — are clearly unsuitable.
Manufacturability and production costs also play a role in the selection of the ideal delivery platform and processing technology. Any robust tool must include these, and possibly other factors too, such as indication, therapeutic area, chemical stability, and thermal stability. Our tool does this. It presents viable alternatives to minimize the chance of false negatives, and it advises against certain technologies to eliminate false positives.
Patheon’s model is not static. As new products come on the market, we incorporate data about their characteristics to revise the tool and thereby improve its ability to predict the best solubility solutions. We also use information contained in new scientific literature to keep the model updated.
By using this tool early in the development process, sponsors can narrow their technology choices to the few with the highest potential for success. That can help them reduce their costs, improve their success rates, and shorten their products’ time to market.
Visit patheon.com/solubilityenhancement to discover the technologies that are most likely to help address your molecule’s low solubility challenges.
