How To Fast-Track Your Oral Solid Dose To Phase 1
By Chukwuma Ude, Associate Director of Pharmaceutical Development at Pace® Life Sciences
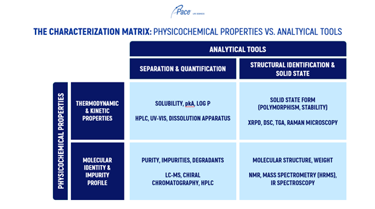
Accelerating a BCS Class II oral solid dosage form into Phase 1 requires a strategic, science‑driven framework designed to optimize bioavailability while conserving limited drug substance. The process begins with defining the molecule’s “DNA” through structural elucidation and understanding key parameters such as pKa, Log P/Log D, and pH‑solubility behavior. With these boundaries established, solid‑state characterization identifies the most appropriate crystalline or amorphous form using tools like XRPD, DSC, and thermal profiling to prevent stability or manufacturability challenges.
Once the optimal form is selected, formulation scientists focus on solubility enhancement and excipient compatibility, creating a micro‑environment that supports dissolution regardless of physiological conditions. Manufacturing strategy is then tailored to API constraints, selecting direct compression, dry granulation, or wet granulation based on flowability, compressibility, and content uniformity requirements. Finally, dissolution performance, forced degradation, and accelerated stability studies verify clinical readiness. By integrating these parallel workstreams, teams can confidently shorten timelines and advance oral solid dose candidates efficiently into first‑in‑human studies.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
