How To Improve Sponsor–CMO Collaboration Around Digital Deviations
By BioPhorum

Managing deviations within the biopharmaceutical industry is a critical process to ensure product quality, regulatory compliance, and, ultimately, patient safety. Today, deviation management between sponsors and contract manufacturing organizations (CMOs) is heavily manual, complex, and fragmented, leading to inefficiencies, data integrity risks, and delayed resolutions. These challenges are exacerbated by inconsistent quality agreements, varying data standards, and the burden of manual data entry across disparate quality systems.
Recognizing these inefficiencies, this article presents a vision for digital deviation management — a standardized, digital-first approach to deviation reporting, data exchange, and resolution. By adopting digital strategies and standardized data models, organizations can significantly reduce manual processing, improve the accuracy and timeliness of information sharing, and strengthen trust between sponsors and CMOs.
This article outlines a clear framework for how the industry can move forward, including the definition of digital deviations, the key benefits of digitalization, and practical considerations such as data transmission alignment, validation requirements, and the use of industry-standard fields and controlled vocabularies.
Digital Deviation Management
Digital Deviations Defined
While each organization strives for an effective and efficient path to compliance with each deviation, every organization has its own interpretation of the regulatory requirements and expectations. This creates a significant challenge when establishing the foundational standards and expectations that are critical in supporting digital deviation management. The standards and expectations must have enough detail to enable consistency across each network of sponsors and CMOs, as well as between them. On the other hand, there must be flexibility to support the unique needs of sponsors and CMOs. The ever-changing compliance landscape will also present challenges in establishing and maintaining standards and expectations, requiring a strong foundation that can be enhanced and revised to accommodate future needs.
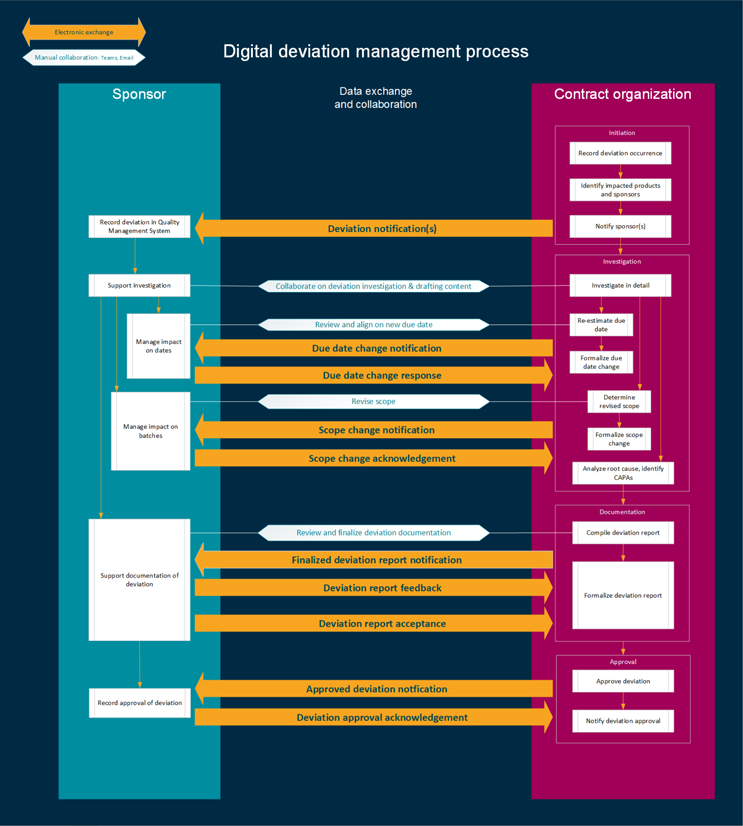
Overall, the need for digital deviations is significant. By leveraging the standards and expectations established within this article for deviation business processes and data, sponsors and CMOs can notify, share, and execute the deviation management process as effectively and efficiently as possible. The direct benefits of digital deviation management focus on reducing the time spent on executing manual steps of the current deviation processes across both organizations (see Figure 1), allowing that time to be repurposed for more value-added actions.
Figure 1: The digital deviation management process. Click on image to enlarge.
Benefits
One of the most critical elements to be scrutinized across the manufacture of pharmaceuticals is divergences from a standard or expected value. Understanding and analyzing data, processes, and outcomes is essential to determining the potential impact on the patient and product. This area is one of the highest priorities in data sharing. It is in the interest of both the sponsor and CMO to determine any potential risk or impact due to a deviation. Driving down the time it takes to close out a deviation in both the sponsor and CMO systems will improve the efficiency of supply, and the transparency and alignment of events and their outcomes are essential to ensuring that what is produced is safe and efficacious for the patient. For these reasons, the timely sharing of information on deviations is a central element of a quality agreement. This is a matter of compliance, demonstrating a thorough understanding and control over the manufacturing process from both the sponsor’s and CMO’s perspective.
Beyond the compliance expectation, sponsors and CMOs have shared goals when it comes to executing digital deviation management. Some of the benefits of digitizing deviations include:
- Improved transparency for sponsors. If a deviation happens at the CMO, the responsibility for investigating and understanding what happened lies with the CMO; therefore, the sponsor realizes the transparency benefit. Increased transparency also means earlier detection of missing information, which can improve the speed of closing out deviations. Reviewing trends or patterns that will prevent deviations can lead to strengthened sponsor/CMO partnerships.
- Having a digital, more digestible format allows organizations to analyze and create a fuller picture of open/closed deviations, etc., allowing the management of resources based on the priority of batches. This can also result in fewer human errors and faster handling as there are no more emails, online collaboration systems notifications, or papers being circulated.
- Reduced time-to-batch disposition, which can lead to shorter end-to-end lead times. Short lead times mean a reduction in safety stock requirements, as these calculations are traditionally based on lead time. This change reduces supply chain risk and inventory costs and, overall, results in improved supply for patients.
Challenges
A key challenge to managing deviations is the difference between the sponsor’s and CMO’s company cultures, perspectives, and approaches to the deviation management process, which can be significant. This challenge exists regardless of the digital maturity of the data exchange and magnifies as organizations progress to higher levels of digital maturity. Organizations exchanging digital deviations are forced to ensure their internal technology systems and processes incorporate their shared requirements.
Building on this challenge, there may be issues around the timing, accuracy, and integrity of data exchanged for digital deviations. Deviations also represent a potential impact on product safety, identity, strength, purity and quality, or compliance with regulatory requirements. Due to the potential severity of these events, digital exchanges of deviation information must be made in a timely manner and adhere to the timing expectations outlined in the quality agreement.
When it comes to data accuracy and integrity, the digital exchange of a deviation notification must accurately reflect the deviation data stored within the CMO’s quality management system (QMS). It must be validated or qualified at a level that matches the intended use of the information shared. The quality agreement must outline the agreed transfer of data ownership and the ownership of validation responsibilities between the sender and receiver of the data.
The electronic exchange of deviation information must not compromise the completeness, consistency, and accuracy requirements, and the data must be attributable, legible, contemporaneously recorded, original (or a true copy), and accurate (ALCOA). The deviation data must also be secure from unauthorized alteration throughout the exchange process. For example, an electronic, encrypted stamp of authentication (a digital signature) on the digital deviation and its corresponding metadata can be used to confirm that the information originated from the sender and has not been altered.
How Do We Digitize The Process?
Data Transmission Alignment
The digital maturity level of the sponsor and CMO will affect the type of transaction and file format to be used. The limiting factor here lies with the company with the lower level of maturity, as the exchange needs to be in line with this level. Although organizations at lower maturity levels will leverage PDFs to exchange deviation information, the minimum recommended approach for automated data ingestion is a flat CSV format. To better facilitate ingestion and accommodate extended data sets, solutions should use a structured data serialization format, such as XML or JSON. The application programming interface (API) should be precisely defined using OpenAPI or AsyncAPI to facilitate implementation on common integration platforms.
Validation Considerations
Deviations management helps us understand how a nonconformance that has occurred can impact product quality. The electronic exchanges of deviation data between the sponsor and CMO, outlined in the digital deviation management process, support this critical quality business process. Each organization must perform the appropriate validation activities for the validation responsibilities it owns, as outlined in the quality agreement. To execute the outlined responsibilities, each organization will follow its computer systems quality assurance (CSQA) policies and procedures. The quality agreement will be used to establish alignment on the extent of CSQA policies and procedures when significant differences in approach exist between the sponsor and CMO.
Industry-Standard Fields For Initial Notification Transaction
This section covers the data set to be used as a standard reference for the deviation notification in the digital deviation management process. Recommendations to consider include:
- Establish filtering criteria: We recommend that the sponsor and CMO discuss the necessary filtering criteria up front, such as relevant products or risk categories, to limit the amount of data being shared and to prevent data leakage.
- Choose a primary key: Given the nature of deviation data and the frequency of updates, it can be tricky to find the appropriate primary key that serves as a unique identifier of the record. We recommend evaluating a combination of fields that can serve this purpose.
The suggested data model below constitutes an overarching, logical layer of data elements that sponsors and CMOs may map to and use without design changes to facilitate the exchange. The data and descriptions contained in Tables 1 and 2 should not be construed as an explicit prescription but rather as a framework that is anticipated to evolve over time.
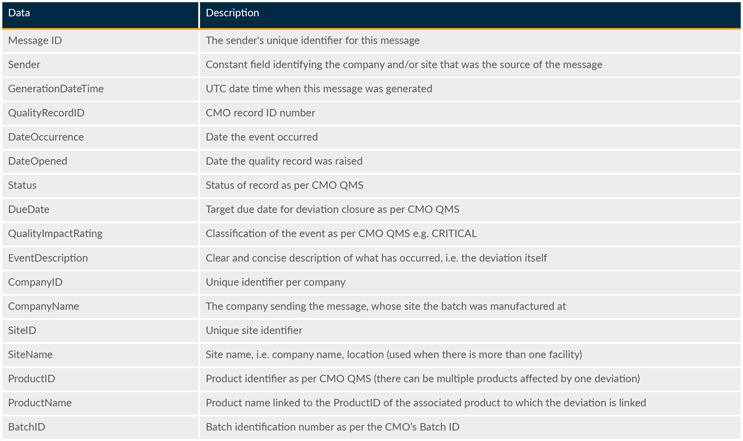
Table 1: Mandatory data
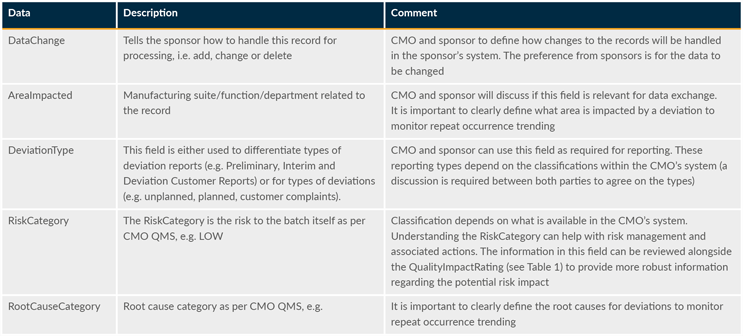
Table 2: Optional data
Controlled Vocabularies
It is not only the names of the fields that should be aligned but also the values that can be contained in those fields. For example, the status descriptor must be the same and mean the same to ensure that the data analyzed from different sources makes sense. Free text should be the exception.
Of course, controlling vocabularies according to an external standard means that both parties in a partnership will need to map to and from their operational systems (rather than the CMO mapping to each sponsor’s requirements). However, once done, that mapping should be good for future partnerships and quicker to implement, with improved accuracy for everyone.
Conclusion
Digitalizing deviation management is not just a competitive advantage but an operational necessity for greater efficiency, transparency, and collaboration between sponsors and CMOs.
This article is intended as a starting point and a catalyst to spark broader industry discussion and action. While the approaches proposed here may not represent the only path forward, they underscore the urgent need to adopt standardized data exchange protocols to reduce manual processes, minimize errors, and ensure the timely resolution of deviations — with significant implications for both supply chain resilience and patient outcomes.
As the biopharmaceutical industry continues to evolve, companies must recognize the importance of digital integration and the critical role of harmonized approaches. Moving forward, stakeholders must commit to aligning on common standards and building on each other’s contributions to work toward one shared goal: a connected, agile, and resilient future for deviation management.
This article summarizes some of the main points from a recent BioPhorum publication on this topic, which includes a case study that demonstrates how digital deviation management can be successfully implemented. To learn more, check out the full paper, Digital deviations: Improving sponsor — CMO collaboration.