Inhale Therapeutics Acquires Particle Technology from Alliance Pharmaceutical
Under the terms of the agreement, Alliance will receive $15 million in cash payments from Inhale and $5 million of Inhale stock. In return, Inhale will be licensed to use PulmoSpheres for respiratory delivery products and will receive $5 million of Alliance stock. On closing of this transaction, Alliance will receive $10 million in cash. Inhale will acquire Alliance's intellectual property portfolio for PulmoSpheres, including several patent applications, and will have the exclusive right to use this technology in the respiratory field.
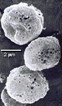
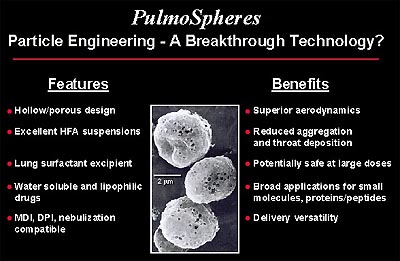
PulmoSpheres uses an emulsification process to produce a powder of hollow, porous, drug-containing shells that are the ideal size for deep lung delivery. When suspended in commonly used propellants or the new environmentally-preferred propellants, PulmoSpheres render stable formulations that could lead to improved efficiency and reproducibility of drugs delivered to the lung via metered dose inhalers (MDIs). Pulmospheres may also work for drug delivery via nasal sprays and nebulizers, as well as dry powder inhalers. Drugs that have been formulated into PulmoSpheres particles include inhaled bronchodilators, anti-inflammatory agents, antibiotics, and proteins and peptides.
"PulmoSpheres technology will help Inhale meet several of its strategic goals," said Robert Chess, chairman and co-CEO of Inhale. "First, it enhances our position as a leader in the pulmonary delivery of macromolecules, such as proteins and peptides. Secondly, it enables Inhale to leverage its dry powder technology platform to address pulmonary delivery opportunities beyond macromolecules, specifically the delivery of a variety of small molecule therapeutics for respiratory and systemic diseases. In addition, PulmoSpheres technology potentially could solve a formulation challenge that currently limits the application of metered-dose inhalers, thus enabling Inhale to address a potentially significant need in this multi-billion dollar market."
Chess explained that PulmoSpheres technology is also attractive to Inhale because the company can use it with only minor modifications to its existing powder processing, filling and packaging systems. This translates into lower infrastructure investment and rapid technology development timelines.
Pulmospheres
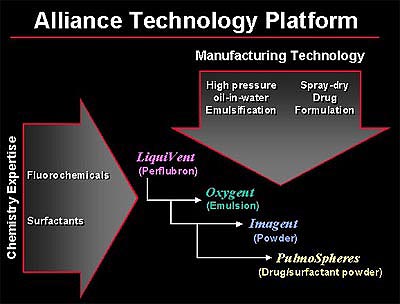
PulmoSpheres are hollow, porous, drug-containing microspheres (in powder form) suspended in perflubron or fluorochemical propellants for the purpose of pulmonary drug delivery. The PulmoSpheres technology is derived, in part, from the combination of various manufacturing processes developed previously for other Alliance products. The formation of PulmoSpheres begins with the mixing of an active drug and a surfactant to form a dispersion. The liquid mixture is then spray-dried to form small, spherical powder particles. The resultant microspheres can then be suspended in fluorochemicals such as perflubron (LiquiVent) or ozone-friendly propellants.
PulmoSphere technology draws on Alliance's expertise in both fluorocarbons and powder technology
After administration into the lungs, the fluorochemical carrier evaporates, leaving the dry, microscopic spheres distributed throughout the lungs. As the microspheres come into contact with the aqueous lining of the lung, they rehydrate and deliver the drug to the tissues. Laboratory and preclinical studies indicate that PulmoSpheres may provide advantages over current formulation technologies with regard to particle suspension stability and flow aerodynamics, which could enhance the efficiency of pulmonary drug delivery.
One potential application for PulmoSpheres is to incorporate asthma drugs in the microspheres for delivery to the lung by means of MDI devices. MDIs typically utilize chlorofluorocarbon (CFC) propellants, but these materials are being phased out due to their implication in stratospheric ozone depletion. The transition from CFCs to more environmentally acceptable products has been difficult, due in large part to the poor solvency of many drugs in non-CFC propellants. No company has yet developed a broadly applicable formulation approach that is able to meet both the demanding criteria of good drug formulation stability and the increasing regulatory standards for MDIs.
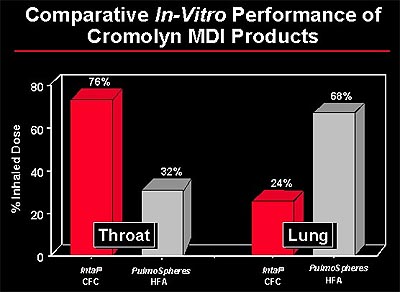
PulmoSpheres could provide a new formulation strategy for MDIs. Their hollow, porous particle morphology allows liquid propellant to permeate within the particles, which improves suspension stability and minimizes particle aggregation. In vitro studies suggest that PulmoSpheres delivered via MDIs may be uniquely efficient in delivering medicaments to the bronchial airways, while limiting deposition in the patient's throat.
About the Companies
Alliance Pharmaceutical Corp. is a research and development company with three products in late-stage human clinical trials: Oxygent, an intravascular oxygen carrier ("blood substitute") intended to reduce the need for donor blood transfusions in surgical patients; LiquiVent, an intrapulmonary "liquid ventilation" agent for treatment of acute lung injury and acute respiratory distress syndrome, and Imagent, an ultrasound contrast agent being developed with Schering AG, Germany.
Inhale develops pulmonary delivery systems to create inhaleable drugs, including peptides and proteins, for systemic and local lung indications. The company has tested six drugs in human clinical trials using its delivery system and has feasibility and development partnerships with several pharmaceutical and biotechnology companies, including Biogen, Centeon, Lilly, and Pfizer. Inhale's most advanced program is inhaleable insulin, which is in Phase III trials with Pfizer Inc. and Hoechst Marion Roussel.
For more information: Christopher J. Searcy, Vice President of New Business Opportunities, Inhale Therapeutic Systems, Inc., 150 Industrial Rd., San Carlos, CA 94070. Tel: 650-631-3100. Fax: 650-631-3150.
