Inspection Of Injectable Products For Visible Particulates
By John Rech, Technology Manager, Particle Testing, and Ravi Patel, Director Quality, Engineering & Metrology
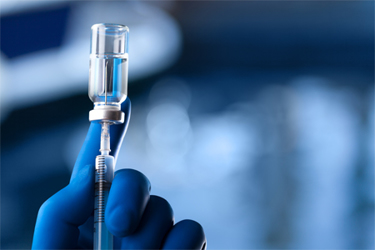
Particulate control is essential for protecting both patient safety and a pharmaceutical company’s reputation. The presence of visible or sub-visible particles is a common reason for recalls, which garner media attention and may be the public’s first association with a drug. Analysis of 2022 FDA recall data shows that from 2018 through 2022, an average of 34% of recalls were due to particulate or lack of sterility attributable to container closure.1,2
In addition, trends in the current pharmaceutical market related to injectable products are driving the need for increased monitoring and control of particulate matter. For example, biologics and other specialized high-value treatments that must be injected are experiencing rapid growth, bringing an increased focus on patient safety and compliance and increasingly stringent regulations related to particulates. Furthermore, recommendations from the Food and Drug Administration (FDA) will focus on systems rather than components and will address the integrity of packaging and delivery systems used with injectables. These recommendations will further drive improvements in particulate control.
Particulate matter in injectables can have multiple origins. This white paper will first examine the risks posed by particulates, the potential sources and types of particulates, and approaches to detect and measure visible particulates in injectables. It will then review the draft FDA guidance3 on inspection of injectable products for visible particulates and discuss how to apply the recommendations.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
