Is Blow-Fill-Seal Right For Your Aseptic Filling Operation?
A Q&A with Patrick Poisson, EVP, Technical Operations, United Therapeutics
 Blow-fill-seal (BFS) is an advanced aseptic pharmaceutical processing technology that is highly automated, capable of producing large batch quantities, and offers a wide variety of container designs and sizes (0.25mL to 1L or larger). The technology integrates plastic blow molding and aseptic filling on a single machine, where the container is created by the machine just prior to filling. The BFS process consists of dry heat extrusion of plastic into a molten tube (i.e., parison), forming of the container in the mold, filling the container with product, insertion of a pre-sterilized component (if applicable), sealing the container, trimming the container, and then conveyance to an inspection and packaging area. Cycle times range from a few seconds to around 20 seconds, depending on type of BFS machine and bottle design and size.
Blow-fill-seal (BFS) is an advanced aseptic pharmaceutical processing technology that is highly automated, capable of producing large batch quantities, and offers a wide variety of container designs and sizes (0.25mL to 1L or larger). The technology integrates plastic blow molding and aseptic filling on a single machine, where the container is created by the machine just prior to filling. The BFS process consists of dry heat extrusion of plastic into a molten tube (i.e., parison), forming of the container in the mold, filling the container with product, insertion of a pre-sterilized component (if applicable), sealing the container, trimming the container, and then conveyance to an inspection and packaging area. Cycle times range from a few seconds to around 20 seconds, depending on type of BFS machine and bottle design and size.
We recently reached out to Patrick Poisson, EVP of technical operations at United Therapeutics, to learn about how his company implemented and uses BFS in its production, what the biggest barriers to further industry adoption of the technology are, and how recent guidance documents — and a new PDA Technical Report — are helping pharmaceutical companies better address those challenges and reap the benefits of BFS in their operations.
What are the primary application areas for BFS technology in the pharmaceutical industry?
BFS has undergone significant evolution since its inception in Europe in the 1960s. The technology was originally used to package food and industrial products, but in the 1970s and early ‘80s it expanded into OTC healthcare and hygiene products. Around that same time, BFS equipment manufacturers began implementing designs that would allow for aseptic manufacture of sterile drug and medical device products, and its use in those areas has grown ever since.
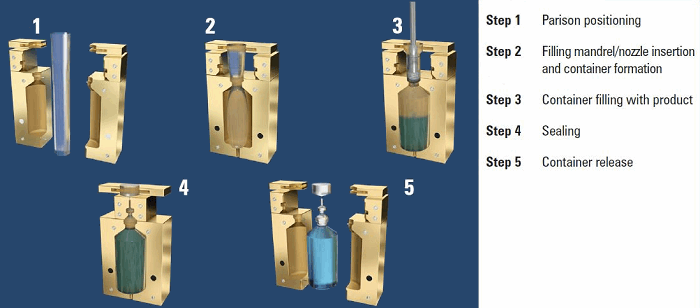
Figure 1: Diagrammatic representation of the open parison BFS process
Today, most people know BFS for its role in manufacturing unit dose inhalation and ophthalmic products. BFS is critically important to the generic inhalation market, especially asthma products, as it is used to cost-effectively manufacture the billions of unit doses that are needed to support that market. Outside of the U.S., BFS is also used to manufacture IV solution containers and some injectable products. In the future, I think you will see some of these types of products get introduced into the U.S. market.
How is United Therapeutics using BFS in its operations?
We use BFS to manufacture a sterile inhalation solution drug product for treatment of pulmonary arterial hypertension (PAH). BFS is the method of choice for chronic-use inhalation products, as the plastic ampoules are a robust container-closure system, are easy to use, and are lightweight to carry. We implemented BFS in the early 2000s during initial product development at a contract manufacturer, which subsequently supported our clinical manufacturing needs. Following commercial approval in 2009, we decided to construct our own BFS facility, which we now use as the primary manufacturing site. As with any start-up, we had some challenges, but we now have progressed to a point where our staff has become very adept at running the equipment.
What were some of those start-up challenges and how did you manage them?
There are always growing pains when you implement a new technology. I knew from past start-up experiences with BFS that operator training, allocation of validation resources, and establishing technology-specific quality systems were going to be key to meeting our internal timeline goals. The BFS machine OEMs do a great job with on-site operator training, and we leveraged that offering to its fullest. I would recommend the same for anyone introducing the technology to their site, as I have seen companies forego this as cost-saving measure only to pay the price later.
Another strategy that we took was involving our manufacturing team with equipment validation. This gave them a deeper understanding of the technology and how it works, and that knowledge continues pay off today. When unforeseen events occur during processing, they often know the potential implications and can take immediate steps to mitigate risk to product quality.
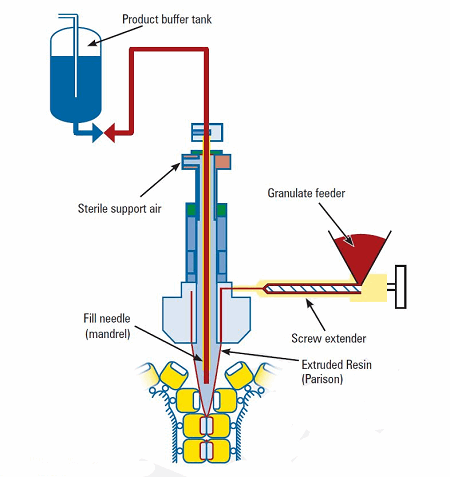
Figure 2: Rotary BFS machine schematic
Finally, it is important to recognize that BFS is a unique technology and that you cannot necessarily apply standards from other aseptic processing technologies without modification. This involves such areas media fills, environmental monitoring.
Why aren’t more pharma/biopharma companies using BFS technology? What are major the barriers to adoption?
BFS has been generally viewed as a niche technology that is complicated to operate. Certainly there is some truth to that belief; however, the reality is that most aseptic filling equipment is complicated to operate. If you have talented people who are willing to be trained, and a management team that is receptive to understanding the nuances of its operation, you can be successful. There are many examples of that throughout industry, including United Therapeutics.
There is also a general lack of knowledge about how the technology works and what its capabilities are. Many people have heard of BFS and know it is used to manufacture plastic containers, but that is where their knowledge ends. Lack of detailed guidance has also contributed, but that has significantly improved over the last 10 to 15 years. The 2004 revision to the FDA’s aseptic processing guidance was a major step forward, with the inclusion of BFS technology as an annex that contains a lot of good information for users. EU Annex 1 started providing direction on BFS in the 1990s and has expanded that direction with each revision. I am anticipating that the next revisions of both guidance documents will continue that trend.
You are an author on the recently released PDA Technical Report No. 77, The Manufacture of Sterile Pharmaceutical Products Using Blow-Fill-Seal Technology. What factors led to the development of that resource?
As mentioned, it has been recognized for many years that there was a lack of written guidance for industry to use as a reference for operation of the technology, and there was a desire to fill that gap. Credit should go to Steve Forrester-Coles, Martin Haerer, Svend-Axel Laursen, Frank Leo, and a few other folks in the BFS industry who, as members of the BFS International Operators Association, had the foresight and commitment in the late 90s to create a Points to Consider document that we were able to use as the basis for the technical report. The PDA subsequently gave us the means to engage industry and regulatory agency reviewers and provide this document to a much a larger audience.
What are the main goals of the report?
The intent was to provide technical information on BFS to current users, potential users, regulators, and others who are interested in the technology. Our goals were to provide specific recommendations on how to implement the technology, how to operate the technology, and how to be compliant with the technology. The content of the report generally falls into those three areas.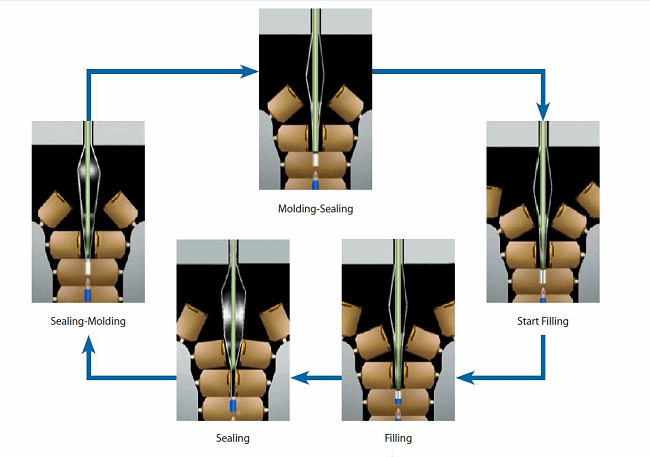
Figure 3: BFS process detail for rotary machines
What are some highlights of the report?
I believe the report is comprehensive in its coverage of the important topics. There is lot of good content at the beginning on design, which encompasses product, machine, and facility design aspects. A majority of the report is focused on operational and qualification considerations; this includes information on critical process parameters, leak detection, environmental monitoring, validation, gowning, and media fills. Media fills are always a hot topic, and with BFS there is interest in understanding strategies for qualifying long fill durations and performing interventions. Risk management is also playing a growing and important role in industry, as driven by ICH Q9, and in support of that we have provided an example of a quality risk assessment, which readers can use as a template to complete their own process/product-specific BFS assessment.
What are the next steps for BFS in the industry?
I think you will see BFS continue to make inroads into the injectable market. BFS machines equipped with insertion capability can place a stopper or rubber septum inside the container prior to sealing, giving the container the same functionality and performance as a glass vial at a much lower cost of goods. Furthermore, these containers are more durable and lighter to ship than glass vials, plus there are no delamination risks. This can be especially important for generic drug makers who are looking for a competitive advantage.
I also believe that there is great opportunity for BFS in drug-device combination products where containers are custom designed to directly interface with a device such as an IV pump or nebulizer — something that really can’t be done with any other aseptic filling technology and that leverages the design flexibility of blow molding. This type of advance will reduce patient manipulation and improve patient safety, as well as provide their innovators with differentiation and possibly intellectual property.
Images used with permission courtesy of PDA from Technical Report 77: The Manufacture of Sterile Pharmaceutical Products Using Blow-Fill-Seal Technology (2017)
