Keys To A Successful Rapid Commercial Launch

INTRODUCTION
Pharmaceutical and biotech companies are spending years and millions of dollars developing drugs or promising new biologics, all with the hopes of saving or enhancing patients’ lives. It’s a “race to the finish” in some cases, and the quicker a company can get its product to patients the better.
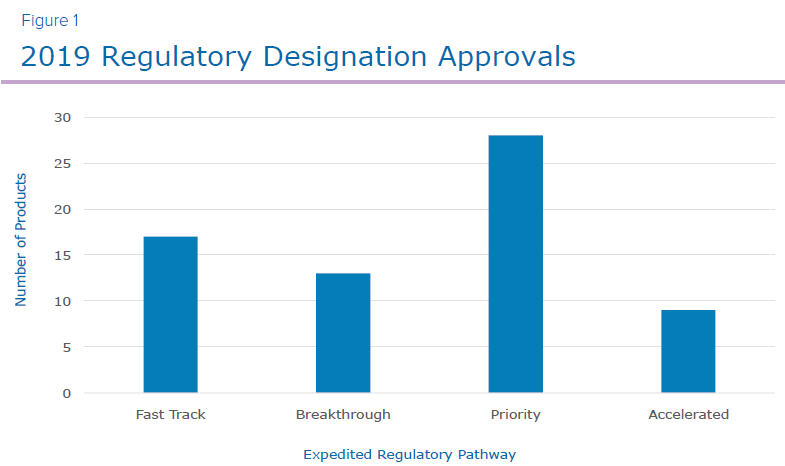
The FDA approved 48 novel drugs in 2019, the third highest number of approvals in over two decades. Of the products approved, 17 were designated as fast track, 13 were breakthrough therapies, 28 were priority review and another 9 received accelerated approval (Figure 1). Sixty percent of the 48 novel drug approvals were designated in one or more of the four expedited categories. With additional novel drug products planned for approval over the next few years along with new and emerging modalities, it is imperative that companies invest adequate time and resources in strategic planning and preparation activities to ensure the best chances for a successful commercial launch.
“Ensuring that the sponsor company and manufacturing partner are aligned is absolutely a key to the success of a product launch. Catalent, having touched nearly 50% of FDA approvals in the last 10 years, has the expertise to bring biologics, small molecules, vaccines, and more to market for drug sponsors,” says Jason Spacek, Vice President, Commercial Operations, North America at Catalent Biologics.

- Agree on Timeline: The customer and launch partner need to agree on a timeline, including the approval of artwork.
- Understand Key Inputs and Relationships: Identify key stakeholders in order to assist with managing information flow and communication between groups to identify and mitigate risks.
- Identify Risks and Create Mitigation Plans: Risk identification may include the approval process for follow-on documentation, necessary validation work, and follow-on production and quality releases. Mitigation plans should be created to anticipate scenarios in which requirements are not met according to the timeline.
- Provide Daily Updates: Daily updates to the project team and customer team are critical to planning the success of a launch. It ensures cooperation and communication between partners and helps identify risks and ensure that mitigation plans are in place.
- Practice Open Communication: Open and accurate communication between partners is key to the success of any commercial launch. You need to have open and straightforward discussions on the project plan in order to move forward and stay ahead of your agreed-upon timeline.
- Understand Roles: It’s important to understand which senior counterparts at both organizations need to be kept appraised, so that if issues need to be escalated, someone at both the sponsor and manufacturer are in a position to help.
Denis Johnson, Vice President and General Manager of Catalent Biologics’ Bloomington, Indiana facility, sees the company forming strategic partnerships to help pharma and biotechs launch products into market, faster. “Often times, a traditional launch may not be what is in the best interest of the patient. If a drug is first-of-its-kind for a life-threatening disease, then Catalent can work with its partners to speed that process up through what we call a rapid launch,” says Johnson.
WHAT IS A RAPID LAUNCH?
Combining a rapid launch following regulatory approval with effective messaging and post-launch services is the key to making your launch successful and gaining a competitive advantage. A rapid launch allows for both the sponsor and the manufacturing partner to work together to provide medicines to patients as fast as possible after regulatory approval.
Catalent Biologics has been an industry leader in driving rapid product launches for a variety of customers over the last decade providing a quick delivery to market, which is imperative in some instances for patients.

RAPID LAUNCH CASE STUDIES
Let’s take a look at real-world examples of rapid launches made possible by Catalent’s expertise and partnership with sponsor companies.
Case study 1: Product launch for a new market within 30 days
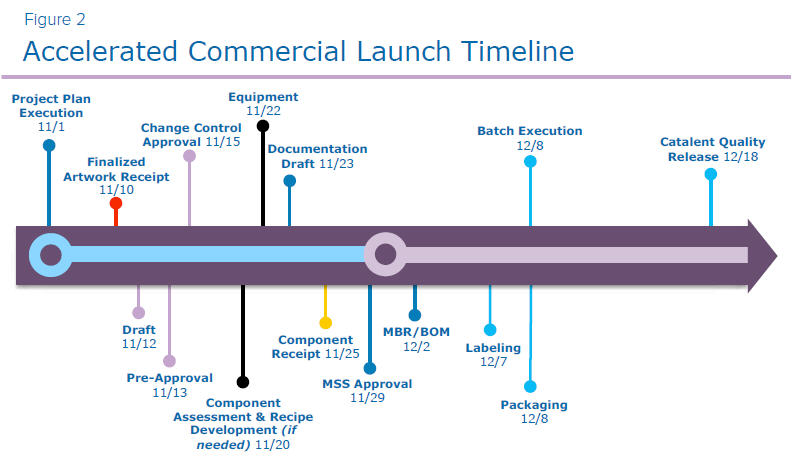
Catalent worked with a drug sponsor that wanted their biologic product to market within 30 days of regulatory approval— not a typical approach for a rapid launch, requiring extremely close communication and collaboration. They required a follow-on product launch of a single product with two SKUs and eight components.

- The true start of the timeline for the project team was the receipt of the finalized artwork. This is the artwork design that has been approved by the region in which the product was launched.
- Next steps involved a series of approval processes, including approval of the draft artwork, preapproval, and the final approval between Catalent, the print vendor, and the sponsor company.
- Catalent requires a change control to document the initial launch to market. With the sponsor’s approval, we may also need to update the artwork to reflect a certain need or requirement within that market.
- If required, we move into validation preparation. Due to different aspects of components or recipe development, various types of validation of engineering work may be required to ensure accurate manufacturing on production day. If required, an equipment protocol is drafted and executed, along with a Process Qualification (PQ) summary report, finalized prior to batch record. However, this project did not require validation preparation.
- The next step was documentation, and giving the order to execute the batch, including Master Batch Records (MBR), material specification sheet, and item masters. These items were bundled and moved through the review and approval process, with both Catalent and the sponsor signing off.
- Next, Catalent actively worked with print vendors to ensure a quick turnaround on print delivery. In most instances, we have a 15-day guaranteed turnaround time from finalized artwork receipt to finalization of delivery to Catalent.
- Upon component receipt, print materials were moved to Catalent incoming quality control for inspection and release for further manufacturing. After release, product moved through labeling and packaging as directed by the approved MBR.
- The final step includes the quality release from Catalent’s site to the customer. Due to the execution timeline, we are typically working through an expedited release, mitigating deviations as we move through the manufacturing process to ensure a quick turnaround on the quality release.
- Once Catalent’s quality release was complete, the batch records were uploaded to a common SharePoint® site, with the sponsor company completing their quality review and release of those batch records before shipping the batch to the next supply node (typically the regional distribution center).

The following steps were taken to accomplish 24-hour rapid launch:
- Catalent immediately started the process of planning with a series of risk-scenario planning exercises, using those tools to clarify with the sponsor what a successful launch would look like from the perspectives of cost, timing, and risk-tolerance.
- The project team moved on to a series of detailed process flows, identifying the activities that needed to happen to make the timeline a reality, and who would be responsible for executing each activity.
- The team looked at which people or groups were responsible for pushing or pulling information through the facility, while also identifying each group that would interact with the product.
- During planning and execution, Catalent’s team gathered and acted upon useful feedback from involved stakeholders. Clear communication channels were established, so in case there was a concern, there was clarity on where and how to get accurate and timely answers.
- Prior to the actual launch of the product, detailed updates and summaries were provided to the cross-functional team and other stakeholders on a daily basis. This practice helped in ensuring frequent touch points and communication around the status of the project.
KNOWN CHALLENGES WITHIN RAPID LAUNCHES
Rapid commercial launches always bring challenges. Here, we take a look at some of the challenges and key takeaways from dealing with possible hurdles that may arise during the process.
- Artwork Approval Delays
- Specification Alignment
- Validation Requirements (if required)
- Component Failure
- Execution (Planning, Machinability, Resources)
- Deviations (Preventing Batch Release)
Artwork approval delays are a common challenge during rapid launches. Each regulatory agency has their own deadlines they are working against when getting artwork approved. Once the regulatory artwork review is completed, the regulatory agency may delay releasing that approval. Artwork approval can take from 24 hours to several weeks. It is important to calculate this risk within your launch mitigation plan.
Another challenge that may come up is the specification alignment. Specifications should be verified by the launch partner before referral for approval. If specifications are not aligned or changed during the agency review process, the artwork must be returned to be rewritten and reapproved, causing further delays.
Validation requirements could also affect the launch date if they were required and not caught in artwork review. These could include a change in the size of the component, a change in the print on a component, or known validation requirements such as a change in serialization.
To mitigate the possibility of component failures, the drug sponsor must focus on the print vendor that provides labeling, inserts, and cartons that the product will be packaged into. When these components pass through incoming inspection, there is a risk of failure. These failures typically require a quality team review to determine if this particular product failure will delay a component from reaching the market.
All these challenges tie into the execution of the launch timeline. Any component failure or validation requirement could delay the planned production date. Machinability or resources could also be another challenge that can be mitigated to ensure on-time execution.
Lastly, deviations, which are items that require additional investigation or documentation prior to batch release, could put the release date at risk.
KEYS TO SUCCESS

Keys to Success: Planning and Risk Management – This includes articulating the problem statement, understanding what good results look like, practicing with a purpose, establishing a basis to measure progress, and investing in appropriate scenario planning.
Challenge 2: Cultural Understanding of Urgency – This challenge may include complacency with the status quo, response time, dealing with information, push versus pull scenarios, and closing the gap between execution and planning.
Keys to Success: Communication and Operating Mechanisms – Practice overcommunication, engage in face-to-face early discussions, celebrate incremental victories, and project a public relations engine effectively.
Challenge 3: Escalation Process – An intense pressure to deliver success, distance from process execution, and a low level of control add to the challenges.
Keys to Success: Governance and Leadership – Utilizing escalation channels effectively, leveraging each organization’s leadership structure as needed, and generating concise, digestible, frequent project reports.
ABOUT CATALENT BIOLOGICS
For more than two decades, Catalent Biologics has built capabilities and experience in development, manufacturing, and analytical services for new biological entities, gene therapies, biosimilars, and antibody-drug conjugates. The company has worked with 600+ mAbs and 80+ proteins, and more than 120 active clinical trials and 13 marketed products have used GPEx® cell line engineering technology. A further 35+ commercially-approved products have employed Catalent Biologics’ capabilities through to drug product fill/finish.
Catalent Cell & Gene Therapy, a unit of Catalent Biologics, is a full-service partner for adeno-associated virus (AAV) vectors and CAR-T immunotherapies, with deep experience in viral vector scale-up and production. Catalent recently acquired MaSTherCell, adding expertise in autologous and allogeneic cell therapy development and manufacturing. Catalent has produced 100+ cGMP batches across 70+ clinical and commercial programs.
Using advanced technology and tailored solutions for clinical through commercial supply, Catalent Biologics brings better biologic treatments to patients, faster.
