Lentiviral Vector Development & GMP Manufacturing

LentiPeak™, our suspension-based, scalable lentiviral vector production platform, enables efficient transition for cell and gene therapies from preclinical stage through clinical development and commercialization with accelerated timelines and reduced manufacturing costs.
The LentiPeak™ Platform
Our proprietary lentiviral vector (LVV) platform is customized to meet your specific development needs.
We offer our partners a suspension-based, scalable lentiviral vector production platform.
Speed
- Streamlined Tech Transfer between PD and cGMP manufacturing with aligned unit operations, equipment, bill of materials and analytics
- Available cGMP capacity without waiting time
- Parallel development with cell processing activities for ex-vivo programs
Control Strategies
- Efficient supply chain management & risk mitigation
- Comprehensive regulatory support package
- Integrated analytical support with panels of established testing methods in-house
Expertise
- Process utilizes a 4-plasmid 3rd gen LV system and a HEK 293 suspension cell line
- Pre-established cGMP documentation and trained technical team
- Proven platform performance with various CAR and TCR constructs at multiple production scales
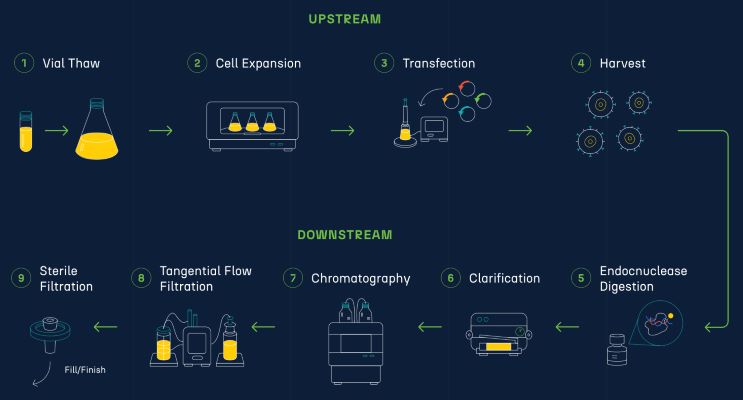
LentiPeak™ Platform: Process Overview
Process
- HEK293 cell line
- Suspension based, serum free
- Transient Transfection
System
- 3rd Generation LV plasmid system
- Accommadates a large range of GOI sizes
- Supporting analytics
Productivity
- High functional titers
- 1.00E+08 to 1.00+09 TU/mL
ElevateBio BaseCamp®
Where the Innovation Happens
BaseCamp is the innovation engine where technology, expertise, operations, processes, and GMP manufacturing come together to overcome industry challenges and accelerate the development of advanced therapies. Click here to learn more about BaseCamp®.