Lyophilization can speed liquids to market

By Donald E. Hagman
N/A
Because liquids are the preferred formulation for commercial-scale production of many parenteral products, lyophilization is of growing importance in bringing liquid products to market. Lyophilization is significant for two reasons:
- increasing numbers of new drug applications
- a decrease in the review times for new drug approvals.
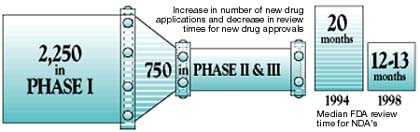
More than 3,000 drug candidates are now in the pipeline in therapeutic areas where parenteral delivery dominates. Of these 3,000 products, about 2,250 are in pre-clinical development or Phase I. The remaining candidates, nearly 750, are in Phase II and Phase III.
Meanwhile, the median FDA review time for NDAs has declined over the last several years. In 1994, the median review time was nearly 20 months. In 1998, it was 12 to13 months. Between the swollen pipeline and accelerated review times, studies project that about 300 new parentals may be approved in the next 10 years, which is twice as many, per year, approved between 1997 and 1999.
Faster time to market
As review time accelerates time to market becomes critical for achieving financial success. This is where lyophilization comes in. Developing liquid and lyophilized products will shorten time to market through the strategy of using lyophilized material for clinical trials while keeping the liquid formulation on stability and continuing to refine it. This way, clinical trials can continue while the preferred commercial dosage form is developed.
Unique requirements
This strategy can and does work, but companies must be highly aware and use extra care when they choose their contract developer and manufacturer.
The best approach is to choose a contract provider that offers both lyophilization and liquid capability, including full scale-up capability in both. Keeping the product with one contractor from development through commercial manufacture offers numerous benefits, especially in terms of quality control, feedback and communication. Choosing a contractor that provides both capabilities also eliminates the high cost—and time delays—of switching facilities.
Expertise
The contract provider should also have the appropriate development and manufacturing services in each area. On an individual product basis, clinical trails can be projected at 5,000 lyophilized units required for Phase I, 10,000 units for Phase II and 50,000 units for Phase III. This makes the contractor's ability to handle small batches effectively and efficiently extremely important. Are they adept at small batch requirements?
Capacity
Small batches add up, however, and providers must have not only ability but also sufficient capacity to deal with them. As the practice of using lyophilization for clinical trial supplies become more prevalent, studies project it will create demand for 4 million lyophilized units per year. Meanwhile, projections put demand for commercial-scale lyophilization at 71 million units by the year 2010. Currently, antibiotic drugs take up a lot of available lyophilization capacity, which, across the industry, has limited capacity for the growing number of biotech candidates in the pipeline.
 It may be wise to look for contractors who are expanding production capacity. They most likely are anticipating your needs and will be prepared to work with them. However, even when all technical and capacity factors are in place, the biggest requirement is always in customer service.
It may be wise to look for contractors who are expanding production capacity. They most likely are anticipating your needs and will be prepared to work with them. However, even when all technical and capacity factors are in place, the biggest requirement is always in customer service.
When shopping for a contractor, whether for clinical supplies, commercial production or both, look for contractors who have a demonstrated customer service orientation, who are equally comfortable dealing with small biotechs and big pharmas. They will share your concern in getting the product to market in the shortest time.
For more information: Arthur C. Solomon, Director of Business Development, SP Pharmaceuticals LLC, 4272 Balloon Park Rd. NE, Albuquerque, New Mexico 87109. Tel: 505-761-9230. Fax: 505-761-9229.
Edited by Angelo DePalma
Managing Editor, Pharmaceutical Online and www.drugdiscoveryonline.com
Email: adepalma@vertical.net
Visit the SP Pharmaceuticals storefront on Pharmaceutical Online.
