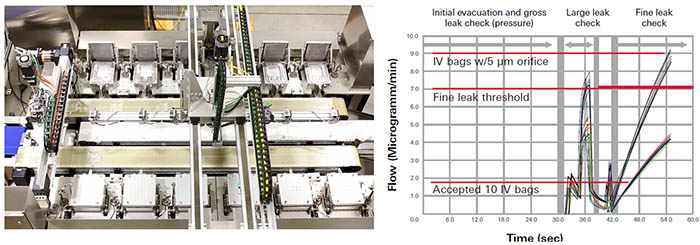
Mass Extraction Integrity Testing for Sterile IV Bags
IV bags are vulnerable to leaks around the seams and especially around the ports where tubes or drugs could be inserted. With our unique USP 1207 recognized Mass Extraction Test Method we offer a deterministic and non destructive test method for this application, which offers various benefits for example in comparison to the “Probabilistic” dye ingress test. “Deterministic” methods are validatable relying on physical measurement technologies where test results are variable and can be compared to pass/fail criteria. Whereas, “Probabilistic” methods, such as dye ingress testing are highly subjective, rely on human interpretation and are prone to error.
In comparison to other non-destructive deterministic methods currently used, the Mass Extraction instruments offer higher efficiency and shorter test time as well as higher accuracy and repeatability. Further benefits of Mass Extraction in comparison to technologies like pressure/vacuum decay or high voltage testing are that Mass Extraction is less sensitive to bag shape and volume, and only requires annual calibration. Thereby it can test multiple bag sizes with one setup.