Nanoparticles for Pharmaceutical Development

By Brian Sulaiman, Dena System BK Ltd.
The pharmaceutical industry has long recognized that smaller particles of active ingredients in formulations improve bioavailability, often resulting in more effective treatments. Dena System BK Ltd. has developed a new technology for achieving exceptional particle size reduction (down to the sub-micron range) while avoiding the contamination that often occurs during traditional processing.
The limitations on new product development imposed by the failure of traditional mixing technologies to achieve dramatic and consistent particle size reduction have now been lifted thanks to Dena System BK Ltd.
Dena's development was boosted by several government awards and by collaboration with one of the world's largest pharmaceutical companies.
Sub-micrometer particles afford multiple benefits to the pharmaceutical industry. Nanometer particles of active ingredients in ointments and creams pass more quickly and effectively into the skin. In tablet form, tiny particles pass through the gut wall easily and treat the affected area quicker that when the particles are larger. As the surface area of the active drug is increased by particle size reduction, a reduced amount of drug is required, leading to reduced adverse reactions and lower manufacturing requirements, resulting in direct cost savings.
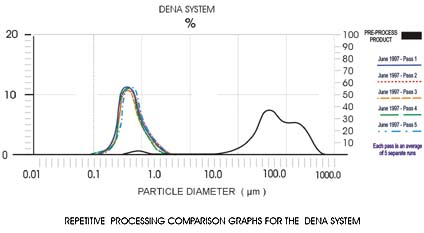
Dena's particle size reduction technology reduces particle size quickly and economically while achieving size reduction in a single pass and avoiding process contamination inherent in other size reduction methods.
Dena works my pumping material slurry through a series of reactors and polishing systems linked by pipework. While larger Dena machines are individually configured to meet a client's output requirements in a single pass, laboratory scale machines use a multi-pass flexible technique with easily changed parameters to determine the output characteristics for the larger systems. That means laboratory scale equipment is easily scaled-up to production scale.
Reactors are at the heart of each Dena system. These have the external appearance of either cylindrical or multi-sided stainless steel prisms. Internally, each reactor performs one or more of the mixing functions: de-agglomerating, dispersing, homogenising or particle size reduction. Suspended within each reactor are numerous elements that flex and recoil rapidly with the introduction of process material.

Dena's particle size reduction system is scalable for lab or production.
Rapid flexing and recoiling creates violent turbulence within a reactor and ensures thorough mixing and de-agglomeration following the numerous particle to particle collisions. Once material passes through the reactors, particles are polished within the special polishing system (SPS). The polished and rounded surfaces on the processed particles achieved within the SPS Milling System inhibits material re-agglomeration.
The combination of special reactors and the SPS milling allow fast, and efficient particle size reduction down to sub-micron scale if required.
Case Study: Dena System Performance Test on ‘Formulation A1' by Durham University
The Dena system was used by a major pharmaceuticals manufacturer during tests on a new formulation. System performance was measured by the size distribution characteristics of resulting batches after different milling periods. All particle size data was provided by a Malvern laser particle sizer package.
Sample Preparation
The material processed, ‘Formula A', was composed of the following:
Methocel E5—50g
Sodium Lauryl Sulphate—2g
Drug—100g
Purified Water—1000ml
For each trial, a pre-milling slurry was prepared by stirring the ingredients vigorously together.
Method
The Dena system used features D3, S2 & SS reactors and the SPS polishing system. It was powered by compressed air at 84psi maximum, the material was pumped through the system by a 1:1 ration diaphragm pump.
Five samples of identical raw material were processed during the trial. One sample of raw material was kept for reference. The samples were processed for a single pass, and 15, 30, 120 & 240 minutes respectively.
Results
The figure below shows the particle size distribution of each sample, after the respective period of milling, the Dena system made the size distribution of the particles significantly narrower. The narrow width of the distribution makes the drug more bioavailable, allowing smaller doses and faster, more accurate ingestion and treatment.
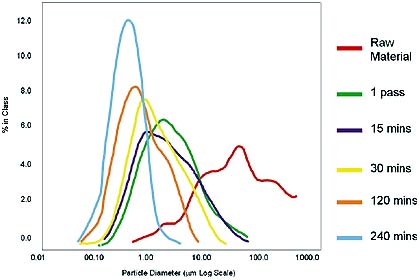
Particle Size Distribution for Dena Milling Trials
Analysis
The Microsoft Excel spreadsheet package was used to analyse the particle size data from the Malvern sizer and to produce Figure 1, Excel was also used to produce standard d10, d50 and d90 measurements, the most pertinent of which are shown below.

Conclusion
After 240 minutes of milling, the Dena system reduced Formula A1 to the indicated particle size range. The extremely narrow size distribution obtained created a 300% increase in the drug's bioavailability, compared to that obtained through conventional milling.
For more information: Brian Sulaiman, Managing Director, Dena System BK Ltd., 14 Mapplewell Park, Wentworth Rd., Mapplewell, Barnsley S75 6DT, United Kingdom. Tel: +44 1 226 388 805. Fax: +44 1 226 383 335.
