Nutraceutix licenses Lehigh Valley Technologies to launch controlled delivery decongestants
Based on patented tabletting process
Nutraceutix Inc. (Redmond, WA) has contracted with Lehigh Valley Technologies (Allentown, PA) to develop two decongestant products for the private label market using Nutraceutix' controlled delivery technology (CDT). Lehigh Valley, a cGMP manufacturer of prescription and over-the-counter (OTC) drugs with expertise in decongestant products, has recently opened a cGMP plant in Allentown. Lehigh Vallen recently concluded an R&D contract with Nutraceutix for these first two products and is presently negotiating for several additional OTC and prescription products utilizing Nutraceutix' patented controlled delivery technology.
The 12-hour and 24-hour decongestants targeting extended relief of sinus and cold symptoms will be launched in mid 2001. A preview of the new products will be available to attendees of the Private Label Manufacturers Show in Chicago, Nov. 12-14, 2000.
The cough and cold market in the United States reached $3.3 billion in sales in 1999, and shows even more promise for the upcoming 2000 cold and flu season. U.S. sales of the two new cough and cold products incorporating Lehigh's controlled delivery are anticipated to reach between $4 million and $7 million in 2002.
Nutraceutix will receive royalties from the Lehigh Valley Technologies sales of these two products commencing in late 2001.
Jeff Moshal, CEO of Lehigh Valley Technologies, said his company was excited about the opportunity "to enter the store brand market with new, unique, patented products." Both companies hope the new formulations will find homes in major retail pharmacy chains such as Wal-Mart, Rite Aid, CVS, Eckert's, and others.
CDT
Nutraceutix developed CDT through a collaboration with Temple University (Philadelphia). CDT was originally targeted to vitamins and supplements, but Nutraceutix believed it would work just as well for pharmaceuticals.
CDT releases medicinals and supplements into the gastrointestinal tract either at a constant rate over an extended period of time, or pulsed at various time intervals. CDT thereby optimizes the active's bioavailability, improves dosing compliance, maximizes efficacy, and minimizes side effects.
The following four panels illustrate the benefits of CDT.
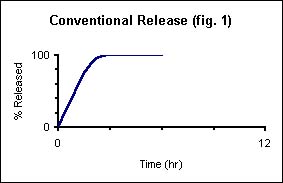
Conventional release of a pharmaceutical from a tablet.

CDT can be used to delay release…

…or deliver a drug in multiple pulses.

CDT may also be used to deliver two or more drugs under different release profiles.
For more information: David Howard, CEO, Nutraceutix Inc., 8340 154th Avenue NE, Redmond, WA 98052-3864. Tel: 425-883-9518. Fax: 425-869-1020.
By Angelo DePalma
Managing Editor, Pharmaceutical Online
