Optimized Glass Primary Containers
Glass plays a crucial role in protecting drug integrity and the performance of any parenteral injection device. By continually perfecting the forming process, we have become a leading producer of high-quality, high-value, ready-to-use, primary packaging for drug delivery systems.
Designed for Drug Delivery Systems: Our Specialty Primary Container Solutions
We offer characterization services for the selection, optimization and integration of primary containers with your device. We also offer ready-to-use syringes and cartridges with up to three times the mechanical resistance of standard glass containers. These high-performance solutions provide a reliable platform for high-force drug delivery devices and support delivery of higher volume and viscous drugs.
Cartridge integration into wearable devices, auto-injectors, and pen injectors
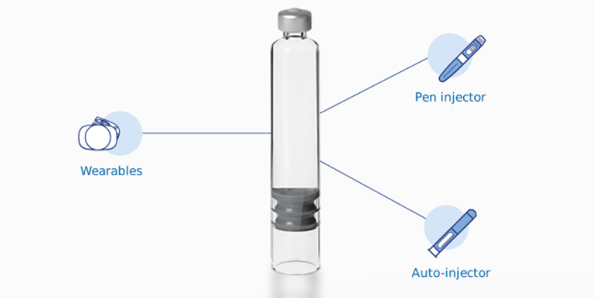
Nexa® 3X glass cartridges: Integrating a glass cartridge into a device has never been so easy
Nexa® 3x Cartridges are suitable for a range of device applications, offering up to 3 times the mechanical resistance when compared to a standard glass container. Thanks to an external anti-friction and anti-scratch silicone coating, Nexa® 3x better withstands the stresses of fill/finish processes too.
We offer
- Characterization of the proper primary container closure system
- 3 mL – 20 mL standard and customized glass cartridges
Syringe integration with auto-injectors
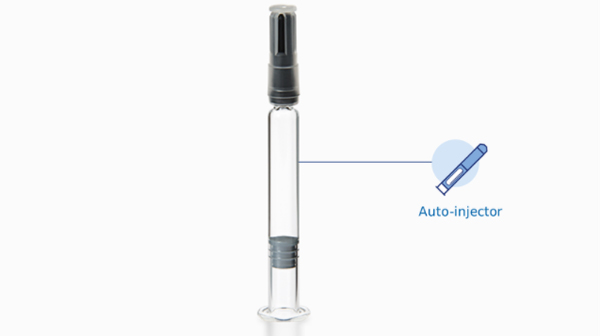
Nexa® Glass Syringe: made to resist
Nexa syringes are specifically designed to meet the dimensional requirements of auto-injectors and to ensure excellent mechanical resistance when compared to a standard syringe. These durable properties are derived through optimized product handling throughout the entire manufacturing process. Advanced in-line inspection technologies carefully check products before release, leading to a minimum risk of in-line breakages during filling operations.
Alba® breakthrough solution for biologics
Optimized for highly sensitive biologics, Alba consists of a range of vials and syringes with consistent chemical and mechanical characteristics. A crosslinked silicon layer provides a consistent interface for complex biologic drugs. Customers can migrate from one container to another with predictable results, de-risking the drug development process when changing container formats from clinical trials to the commercial phase.
We Offer
- Characterization of the proper primary container closure system
- 1mL, 2.25mL and custom pre-fillable EZ-fill® syringes
Microvials for respiratory applications

Our glass micro-vials are produced from Type 1 glass, with a variety of different designs for full compatibility with spray devices. This includes single and multi-dose nasal or sublingual applications
- Custom single or bi dose microvials
EZ-fill®: The market standard for the aseptic manufacturing
EZ-fill® containers are now the market standard reference and are at the core of many complex drug delivery systems. We also customize cartridges, syringes, barrels and micro-vials for special applications.
Key Benefits of our Glass Primary Packaging Solutions
- High performance through accurate production methods and industry-leading dimensional controls & cosmetic inspection
- Reduced time-to-market from clinical to product launch through our ready-to-fill product lines
- Drug delivery systems compatibility and reliable performance for the end-user, ensuring accurate dosing and low dead-volume
- Increased traceability through industry-leading track & trace solutions for fill & finish operations
